sur ABSCIENCES (EPA:AB)
AB Science provides an update on the application for authorization of masitinib in ALS
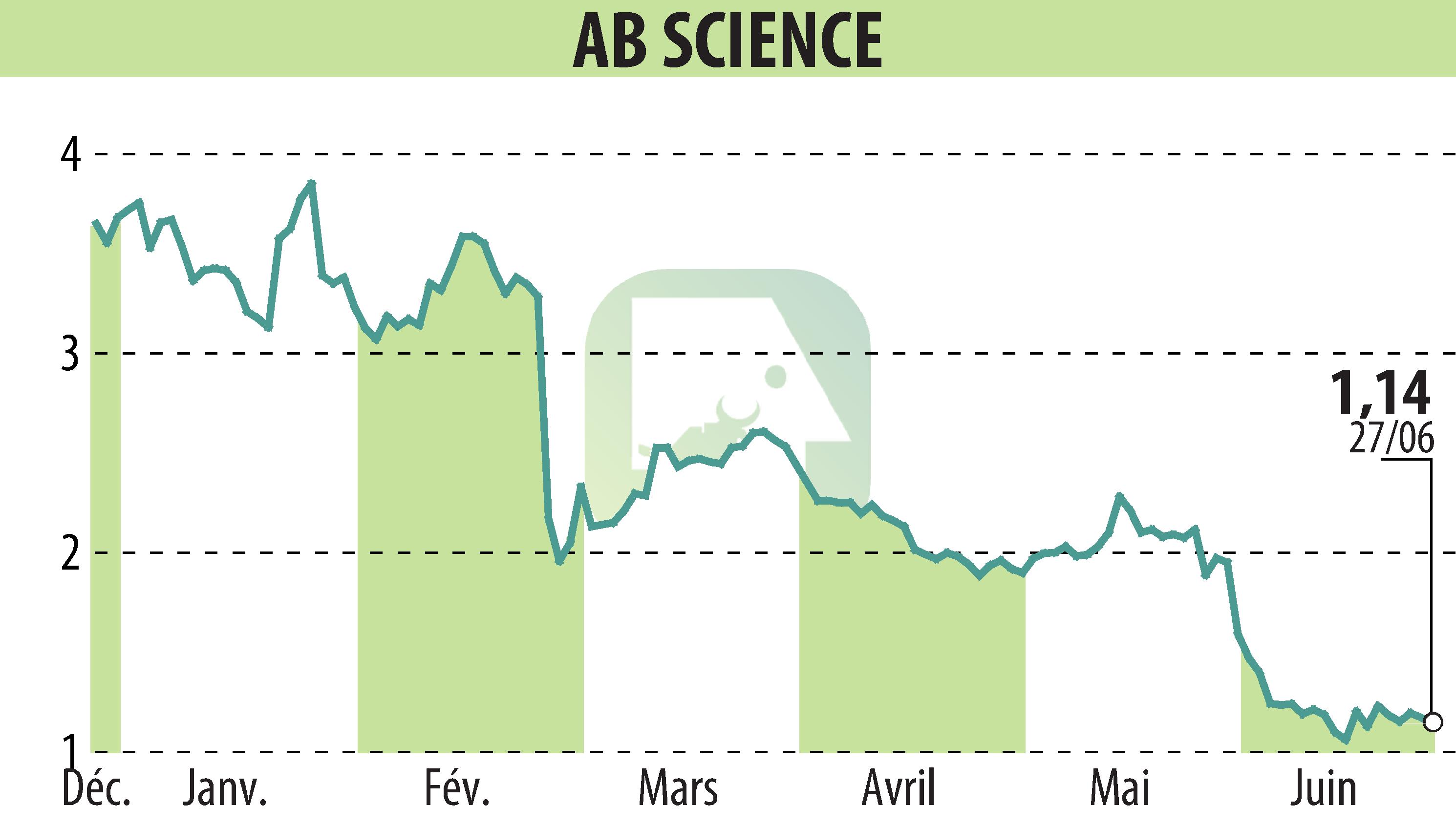
AB Science SA has announced a negative opinion from the Committee for Medicinal Products for Human Use (CHMP) for the conditional marketing authorization of masitinib to treat amyotrophic lateral sclerosis (ALS). The company plans to request a re-examination of the file, highlighting the urgency for patients and the opportunity for a new examination by a “Scientific Advisory Board”.
The CHMP considered the tolerability of masitinib acceptable, but raised objections regarding deviations from Good Clinical Practice and the exclusion of rapid progressors. AB Science responded to these objections by showing the absence of impact of the deviations and by justifying the amendment of the protocol to have a more homogeneous population.
Additional analyzes demonstrated the robustness of the primary analysis, and the subgroup of patients without initial functional loss showed significant positive results, including a 12-month increase in survival. AB Science remains cautious about the outcome of the requested review.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABSCIENCES
