sur AMOEBA (EPA:ALMIB)
Amoéba validates the effectiveness of its biofungicide solution after EFSA approval
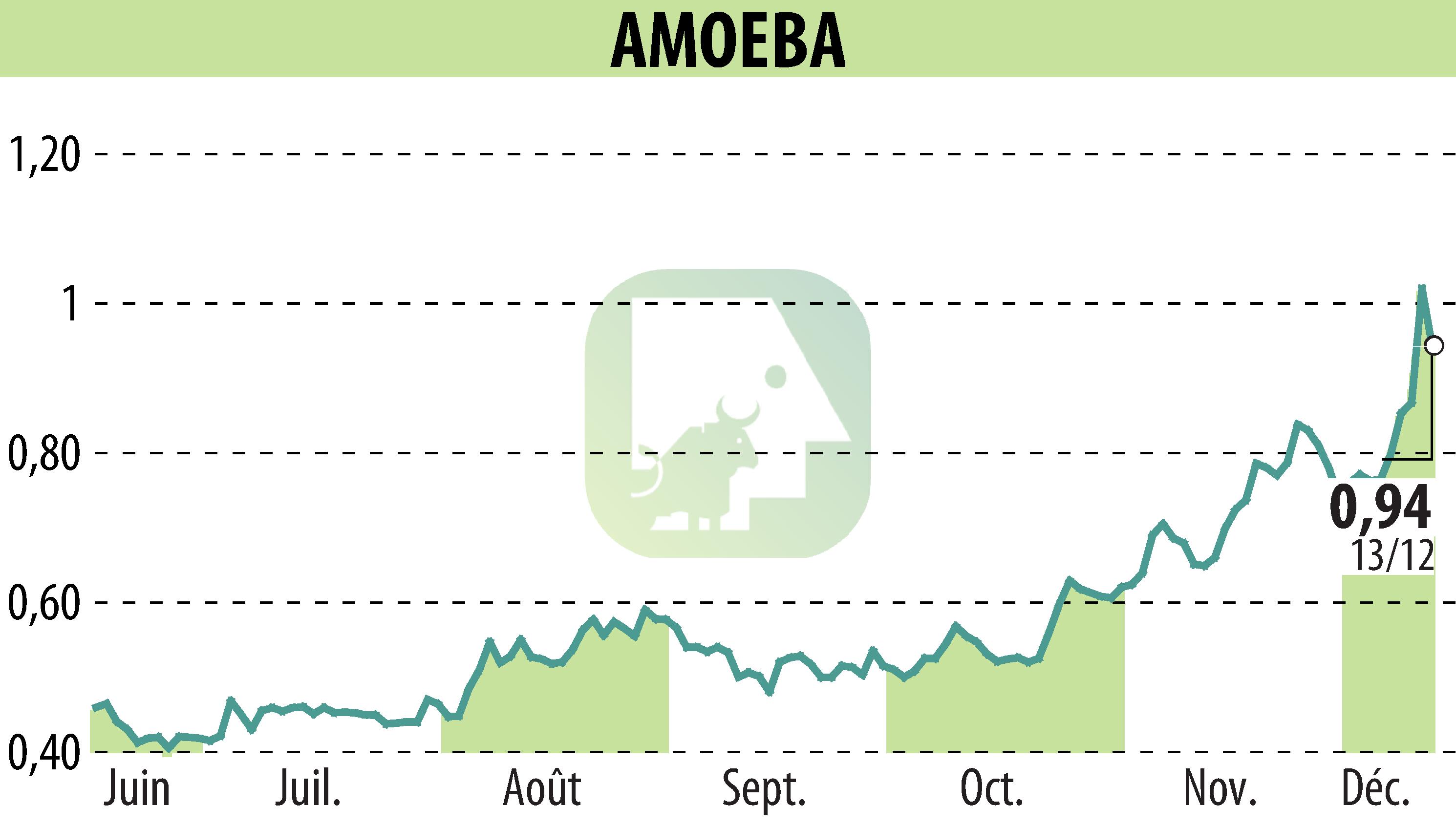
Amoéba has reached a crucial milestone with the EFSA approval of its biocontrol active substance. The report confirms the efficacy and low-risk profile of this substance, which is essential for the AXPERA biofungicide innovation developed by Amoéba. This European validation follows the signing of a Memorandum of Understanding with Koppert, the world leader in biocontrol.
EFSA delivered the final conclusions on 13 December 2024, confirming the substance's strengths in terms of efficacy and environmental safety, without requiring maximum residue limits. Amoéba will thus be able to begin approval procedures in several European markets.
The agreement with Koppert aims to facilitate the commercial and industrial deployment of Amoéba. The outlook paints a promising future for the company, anticipating market entry in 2025.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de AMOEBA
