sur BIOSYNEX (EPA:ALBIO)
BIOSYNEX Obtains Its First CE Markings According to IVDR Regulations
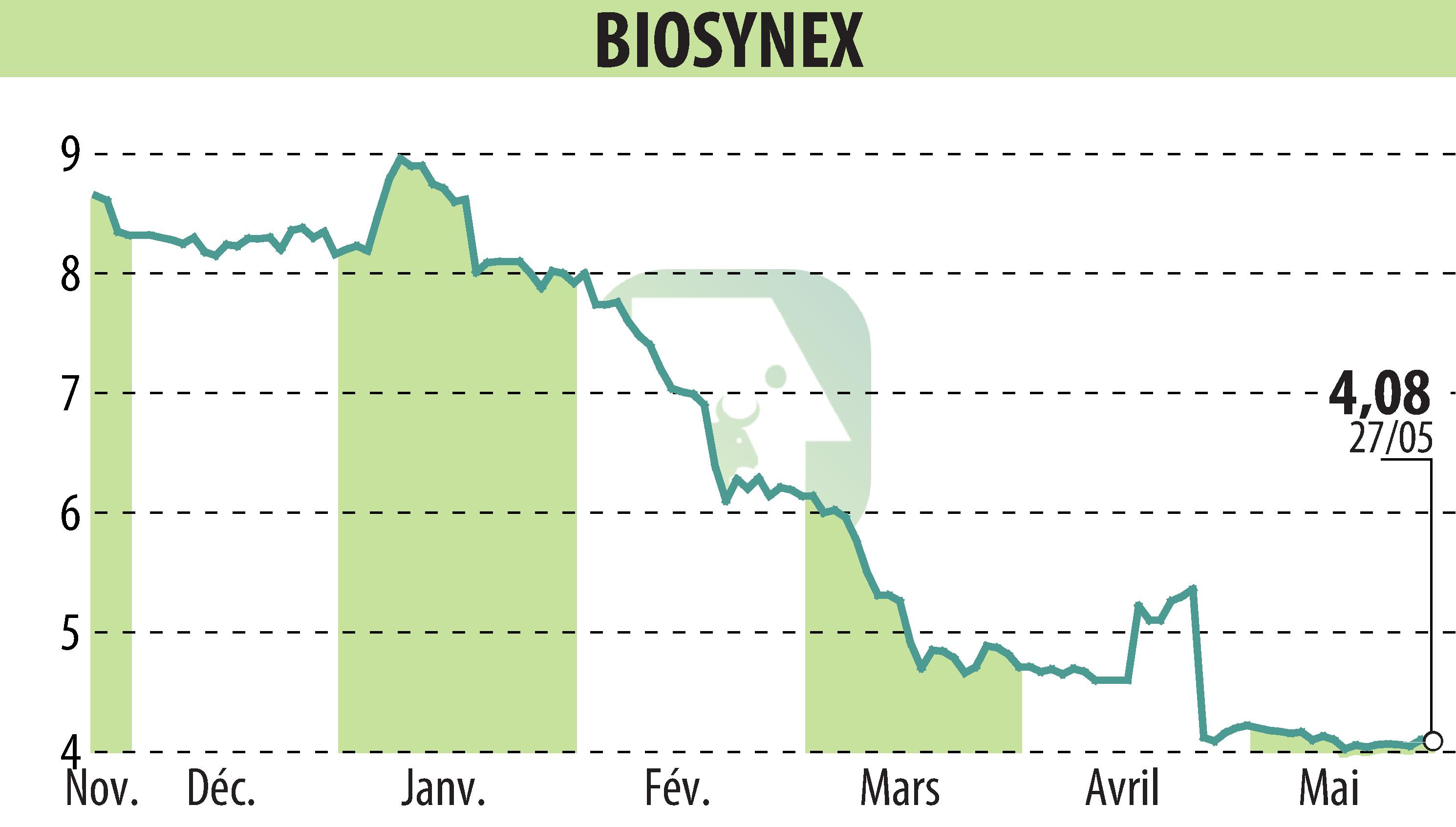
BIOSYNEX, specialist in rapid tests and molecular biology, announced that it had obtained its first CE markings according to the new IVDR regulations. This step marks a significant advance for the safety and effectiveness of in vitro diagnostic devices.
These markings concern Class B and C tests for the detection of infectious agents, including sexually transmitted agents, and a coagulation test developed by Avalun. Compliance with IVDR simplifies certification of similar products in the future.
Products not yet certified continue to operate under the previous Directive until 2027. Recent ISO certifications of BIOSYNEX's quality management system reinforce this regulatory compliance.
The American subsidiary Chembio Diagnostics also obtained CE IVDR marking for a rapid HIV detection test. This compliance and certifications are important competitive assets for BIOSYNEX in a strict regulatory environment.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de BIOSYNEX
