sur DBV TECHNOLOGIES (EPA:DBV)
DBV Technologies: Progress for Viaskin Peanut and financial results for the first half of 2024
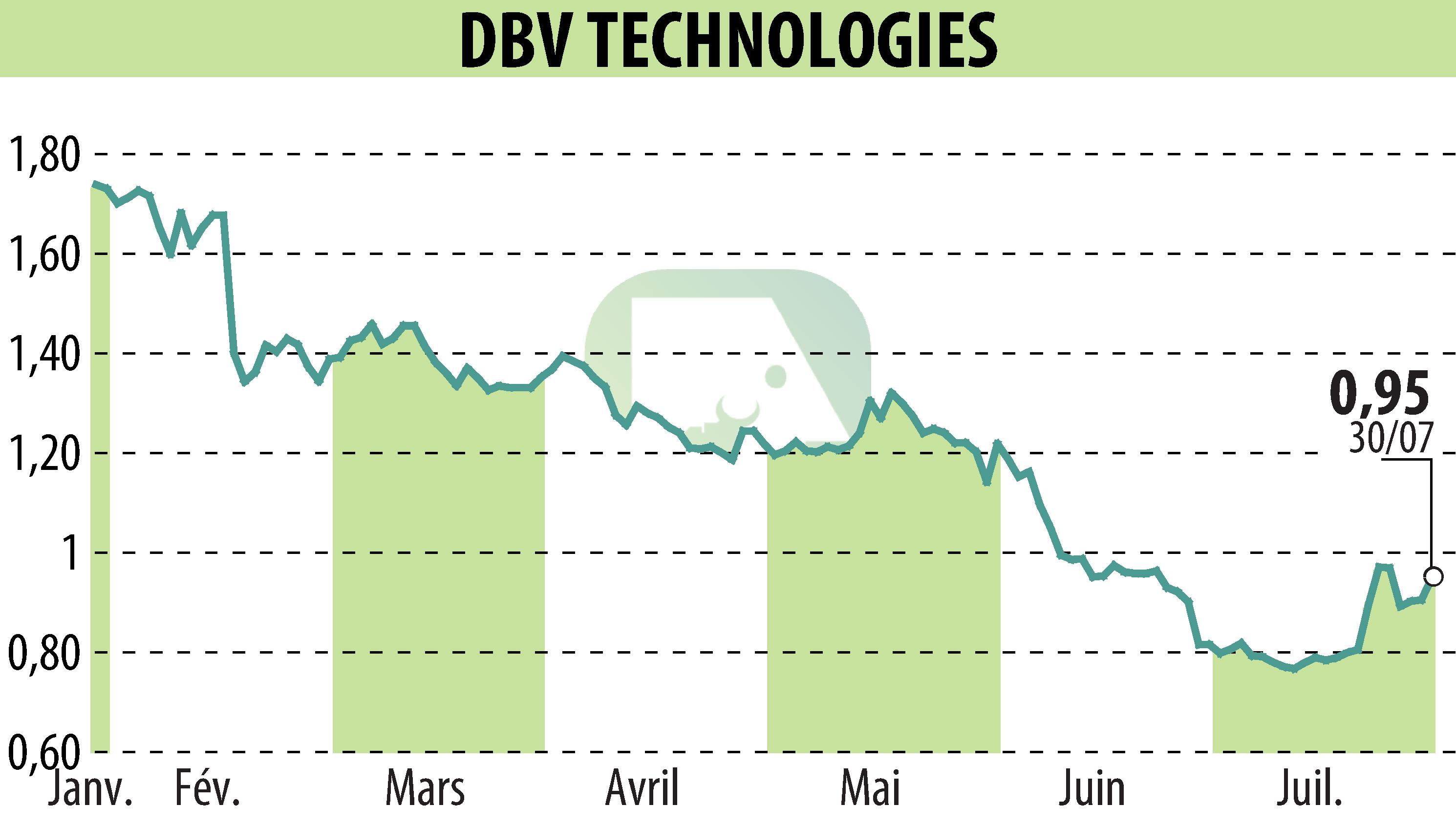
DBV Technologies, a biopharmaceutical company, announces advancements in the Viaskin Peanut program for children ages 4 to 7 and toddlers ages 1 to 3. Recruitment of patients in the VITESSE study, targeting allergic children aged 4 to 7 years, ends in the third quarter of 2024. In addition, the company has proposed to the FDA a new wording for the Viaskin Peanut indication in 1- 3 years, based on EPITOPE efficacy data.
DBV Technologies closes the second quarter of 2024 with cash of $66.2 million. These funds should be sufficient until the first quarter of 2025 thanks to strict savings measures. The company reports a net loss of $60.5 million in the first six months of 2024, an increase from $44.8 million in the same period in 2023.
Operating revenue for the 6-month period ending June 30, 2024 reached $2.6 million, compared to $4.5 million in 2023, due to a reduction in the research tax credit in France. Operating expenses increased by $14.3 million, reaching $65 million, notably due to Research and Development expenses.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de DBV TECHNOLOGIES
