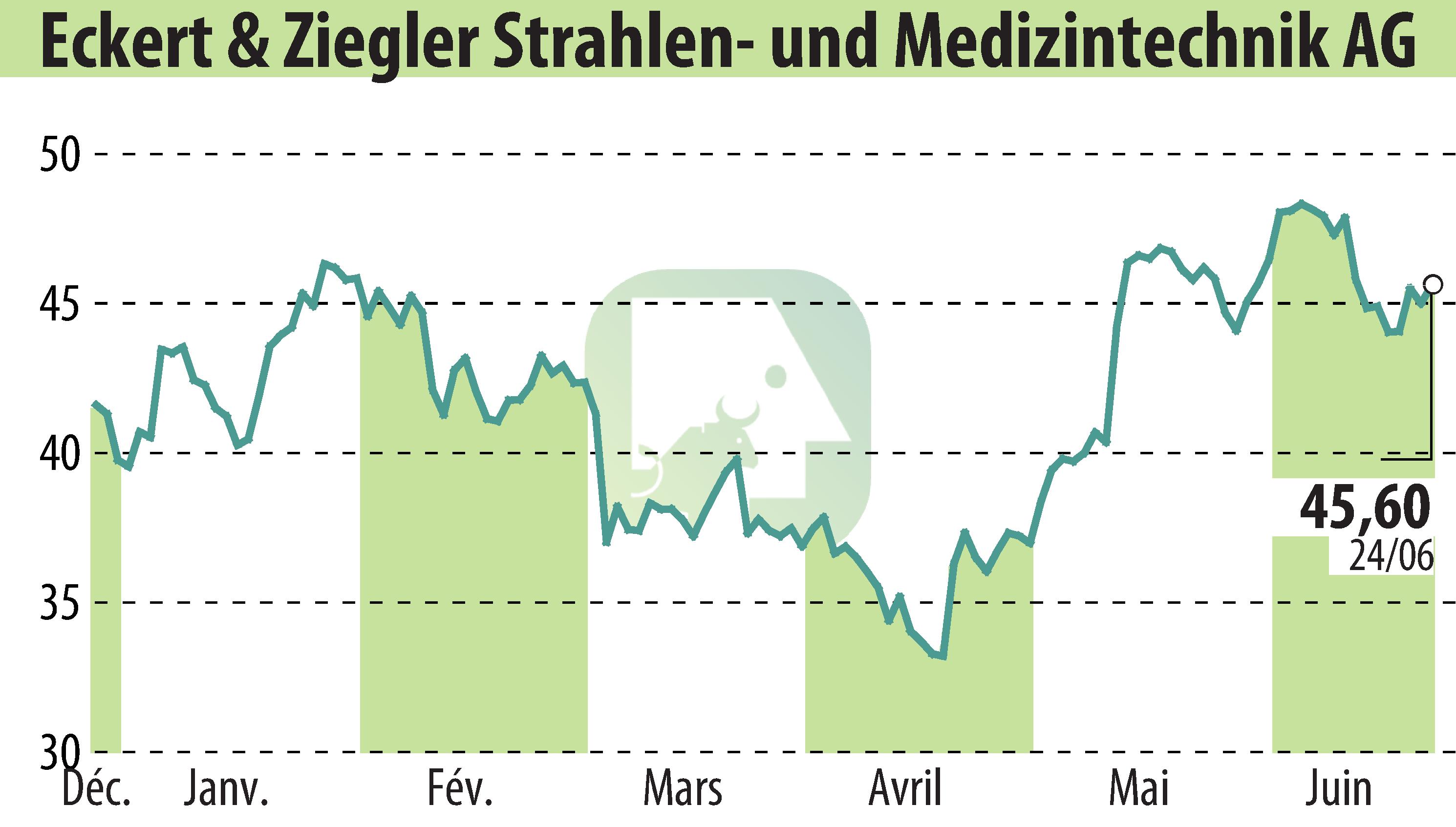
sur Eckert & Ziegler Strahlen- Und Medizintechnik AG (isin : DE0005659700)
Eckert & Ziegler Subsidiary Pentixapharm Receives FDA Feedback to Initiate Phase III Trial with PentixaFor
Pentixapharm AG, a company owned by Eckert & Ziegler SE, has received positive feedback from the FDA to start a Phase III trial with the radiopharmaceutical Ga68-PentixaFor in Primary Aldosteronism (PA). PA, known as Conn’s syndrome, is a common cause of secondary hypertension.
The feedback from a recent Type C meeting suggests that existing clinical data may support the trial, potentially eliminating the need for a second clinical investigation. Ga68-PentixaFor meets criteria for fast track and breakthrough designation, accelerating its investigational new drug (IND) application.
Ga68-PentixaFor is a PET imaging tracer for detecting aldosterone-producing adenomas in PA patients. The approval could provide a non-invasive alternative to adrenal venous sampling, which is the current standard but is invasive and complex.
Dr. Dirk Pleimes of Pentixapharm stated that this milestone enables them to proceed with a US-centric Phase III trial. The aim is to improve diagnostic accuracy in Primary Aldosteronism and patient outcomes.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Eckert & Ziegler Strahlen- Und Medizintechnik AG
