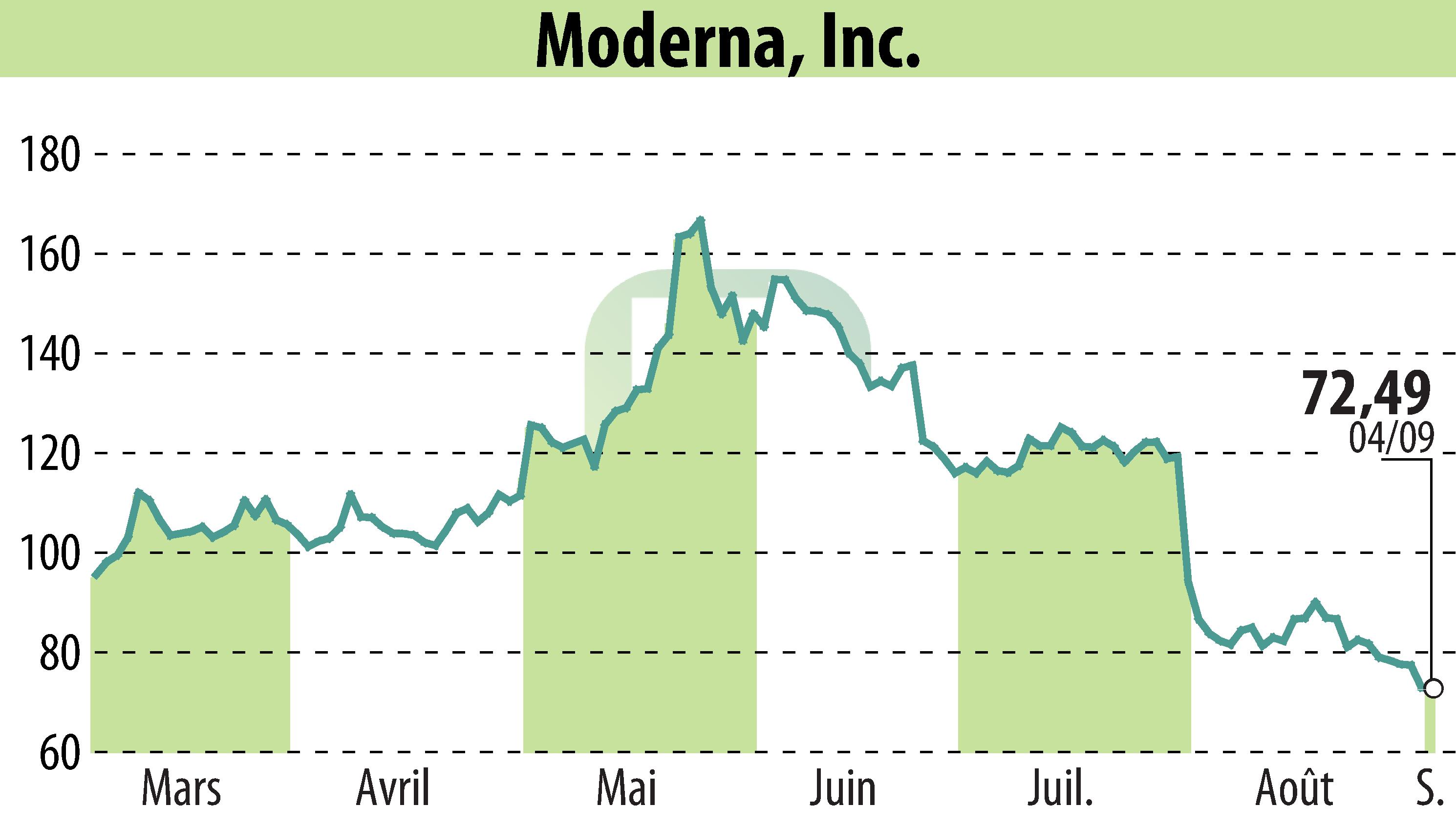
sur Moderna, Inc. (NASDAQ:MRNA)
EMA Recommends Authorization of Moderna's Updated COVID-19 Vaccine Targeting SARS-CoV-2 Variant JN.1
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending the marketing authorization for Moderna's updated COVID-19 mRNA vaccine, targeting the SARS-CoV-2 variant JN.1. This updated formulation, known as Spikevax, aims to provide active immunization against COVID-19 for individuals aged six months and older.
Pending a decision from the European Commission, the vaccine will be available for the 2024-2025 vaccination season. Moderna's CEO, Stéphane Bancel, emphasized the importance of updated vaccines to enhance protection during the winter months when respiratory diseases are more prevalent.
The CHMP's decision is backed by comprehensive data, including manufacturing and preclinical results, and previous clinical and real-world evidence. The updated vaccine composition follows EMA's Emergency Task Force guidance, which recommended targeting the JN.1 family of Omicron subvariants for the 2024-2025 campaign.
Moderna has already received approvals for its JN.1-targeting vaccine in Japan, Taiwan, and the UK. Regulatory applications are also under review in other regions. The company is participating in the European Union's tendering process for mRNA COVID-19 vaccines conducted by the Health Emergency Preparedness and Response Authority (HERA).
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
