sur MEDESIS PHARMA (EPA:ALMDP)
Encouraging results from NanoLithium trial on Alzheimer's disease
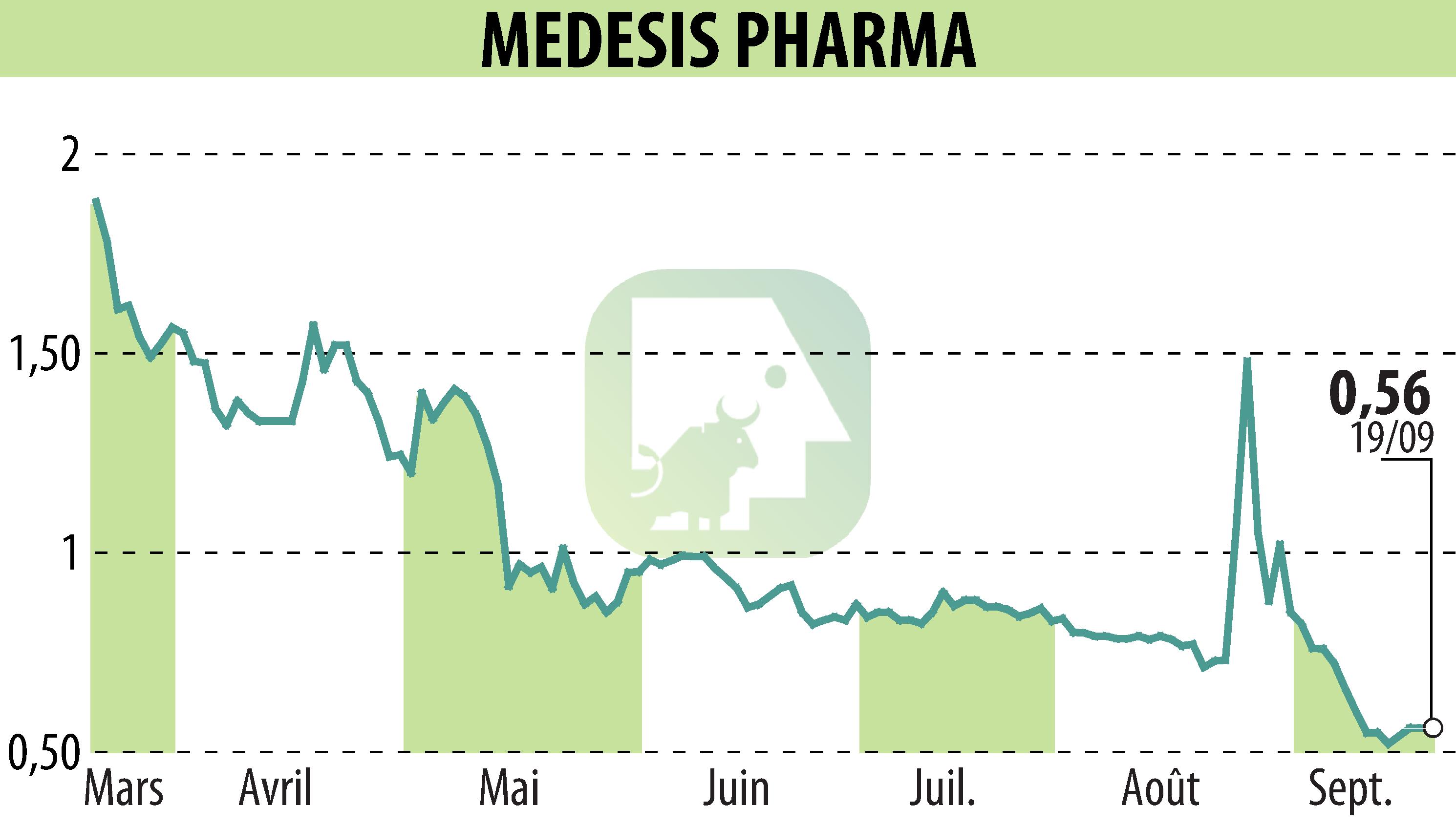
MEDESIS PHARMA announces positive results for the first phase of its phase 2a clinical trial on NanoLithium. The study, conducted on 68 patients with Alzheimer's disease, demonstrates good tolerance and significant clinical improvements compared to placebo.
In a commentary, Dr. Jean-Claude Maurel, CEO of Medesis Pharma, highlights the absence of notable side effects and a greater improvement in patients than that observed in the placebo group. Professor Jacques Touchon supports the continued development of NanoLithium to treat psycho-behavioral disorders associated with Alzheimer's disease.
The study was conducted in a double-blind fashion in eight centers in France, and the results will be followed by a second phase currently underway. Medesis Pharma is preparing to continue discussions with industrial partners for future regulatory clinical studies.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MEDESIS PHARMA
