sur Jaguar Health, Inc. (NASDAQ:JAGX)
FDA Grants Orphan-Drug Designation to Jaguar Health's Crofelemer for Cholera
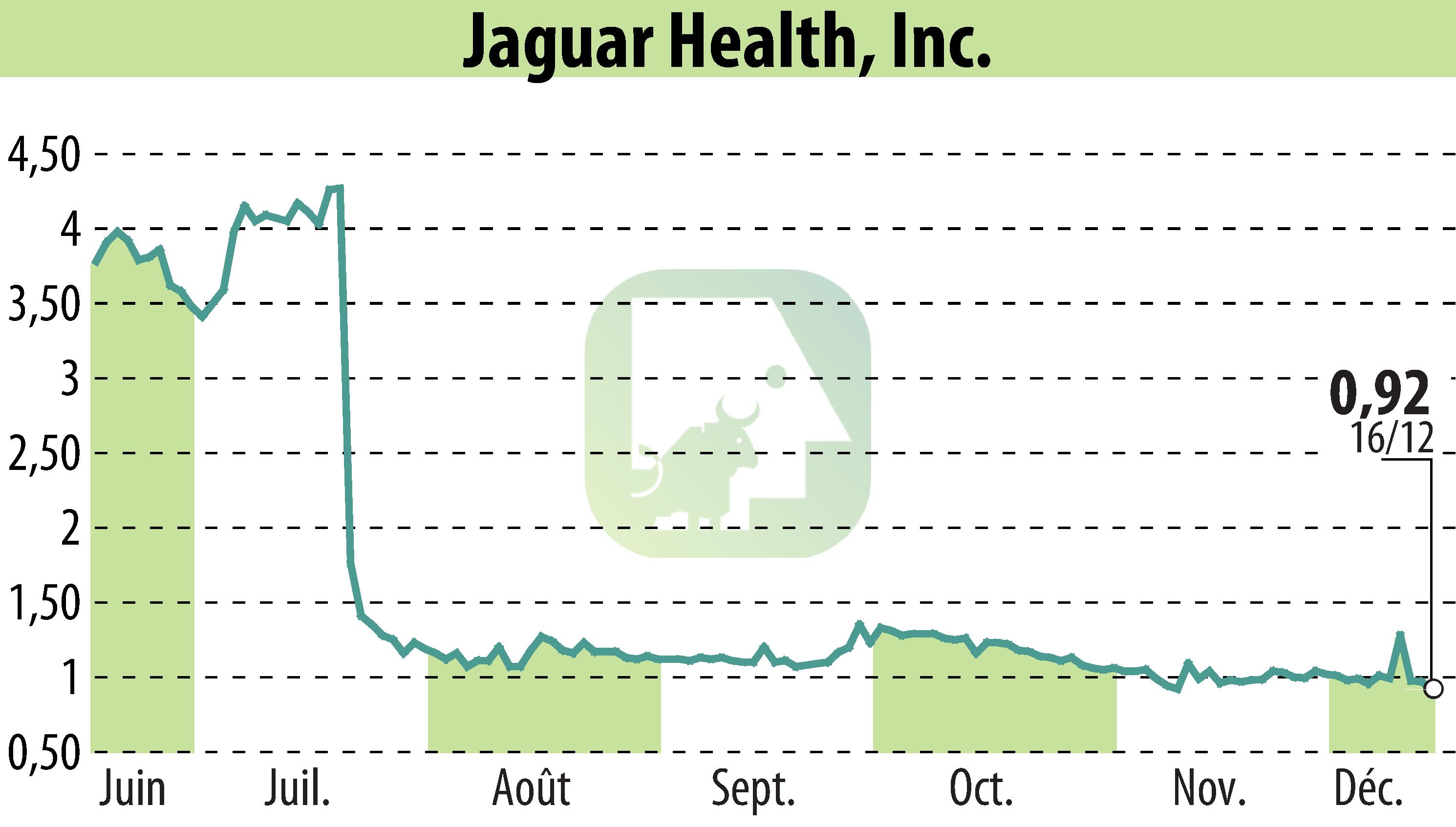
The U.S. Food and Drug Administration (FDA) has awarded orphan-drug designation to Jaguar Health's Crofelemer for treating diarrhea in cholera. Cholera, an acute diarrheal illness, presents a significant health challenge globally, with millions of cases and thousands of fatalities annually. The designation offers Jaguar Health various developmental incentives, including potential tax benefits and a seven-year marketing exclusivity upon approval.
Currently, Crofelemer is also involved in studies targeting other rare diseases. Jaguar aims to extend its orphan-drug pursuit for Crofelemer in Europe and consider a similar designation for its NP-300 compound. Amidst a backdrop of rising global cholera cases, this development represents a strategic advancement in addressing this health crisis.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
