sur INNATE PHARMA (EPA:IPH)
Innate Pharma Initiates Phase 1 Study of IPH4502 for Advanced Solid Tumors
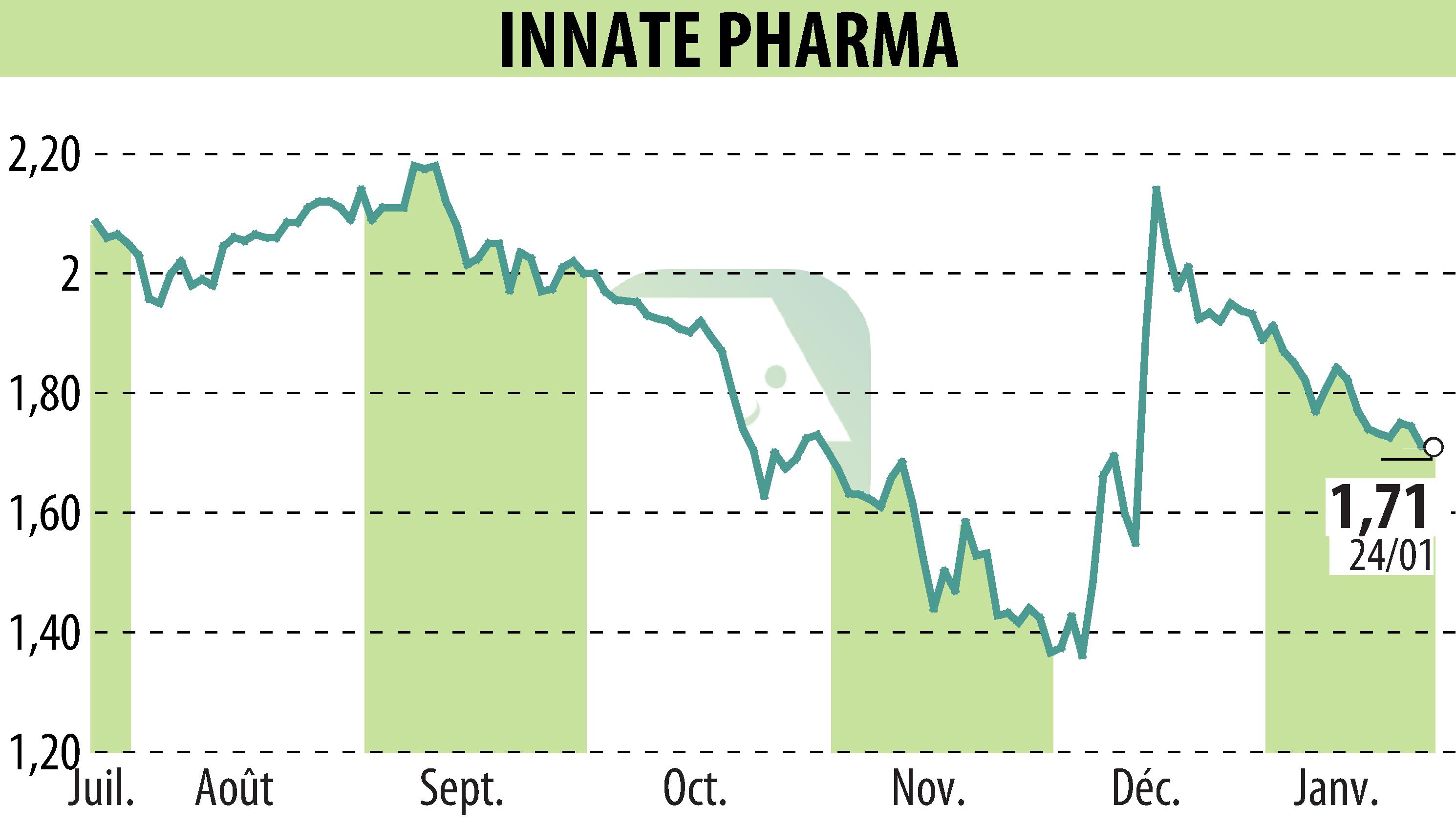
Innate Pharma SA has commenced dosing in a Phase 1 trial of IPH4502, targeting Nectin-4 in advanced solid tumors. This study marks a key development for Innate's clinical pipeline, focusing on the ADC IPH4502, a topoisomerase I inhibitor conjugated to exatecan. The ongoing trial targets diverse tumors, including urothelial carcinoma and non-small cell lung cancer, expressing Nectin-4.
The trial, registered under NCT06781983, aims to assess the safety, tolerability, and initial efficacy of IPH4502. Approximately 105 patients will participate in the open-label, multi-center study featuring two parts: Dose Escalation and Dose Optimization. The study's initiation is a significant milestone spotlighting IPH4502's potential in treating advanced tumors resistant to existing ADC therapies.
Dr. Shiraj Sen and Dr. Sonia Quaratino of Innate Pharma expressed optimism about improving therapeutic options for patients with limited treatment avenues. Further details on the trial are available on clinicaltrials.gov.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de INNATE PHARMA
