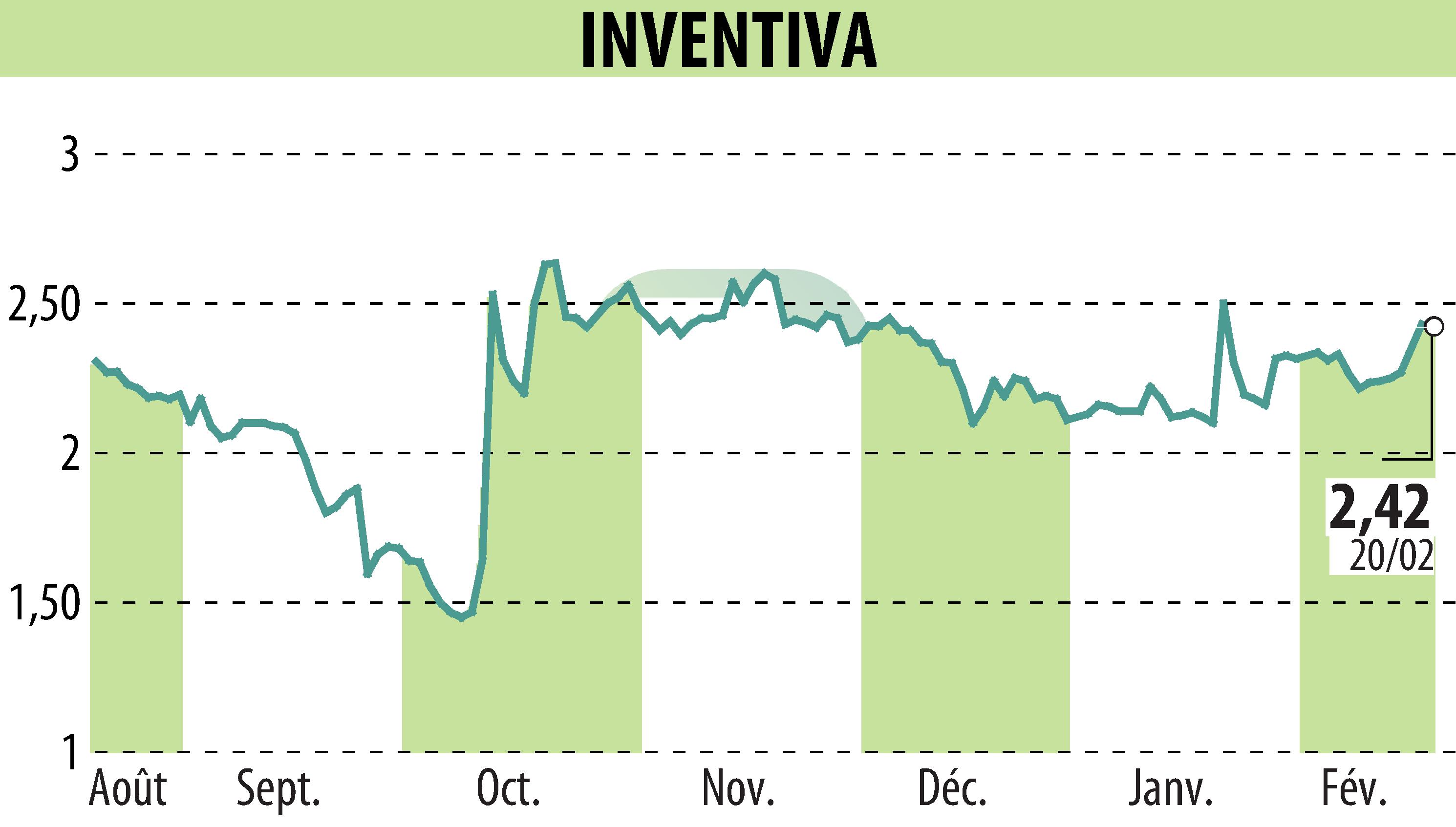
sur INVENTIVA (EPA:IVA)
Inventiva launches clinical development of lanifibranor in Japan
Biopharmaceutical company Inventiva and its Japanese partner Hepalys Pharma, Inc. have launched a clinical development program for lanifibranor in Japan. This project begins with a Phase 1 study that has enrolled its first participant. This study will evaluate the safety, tolerability, as well as the pharmacokinetic and pharmacodynamic aspects of the drug.
This development is part of a strategic partnership established in 2023 between Inventiva and Hepalys for the commercialization of lanifibranor in Japan and South Korea. The positive results of this study could allow the advancement to a Phase 3 study for Japanese patients with MASH, a liver disease.
This initiative marks an important milestone for both companies in their efforts to treat steatohepatitis associated with metabolic dysfunction in Japan, where up to 2.7% of the population is affected.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de INVENTIVA
