sur INVENTIVA (EPA:IVA)
Inventiva: update on the NATiV3 clinical program and financial statement
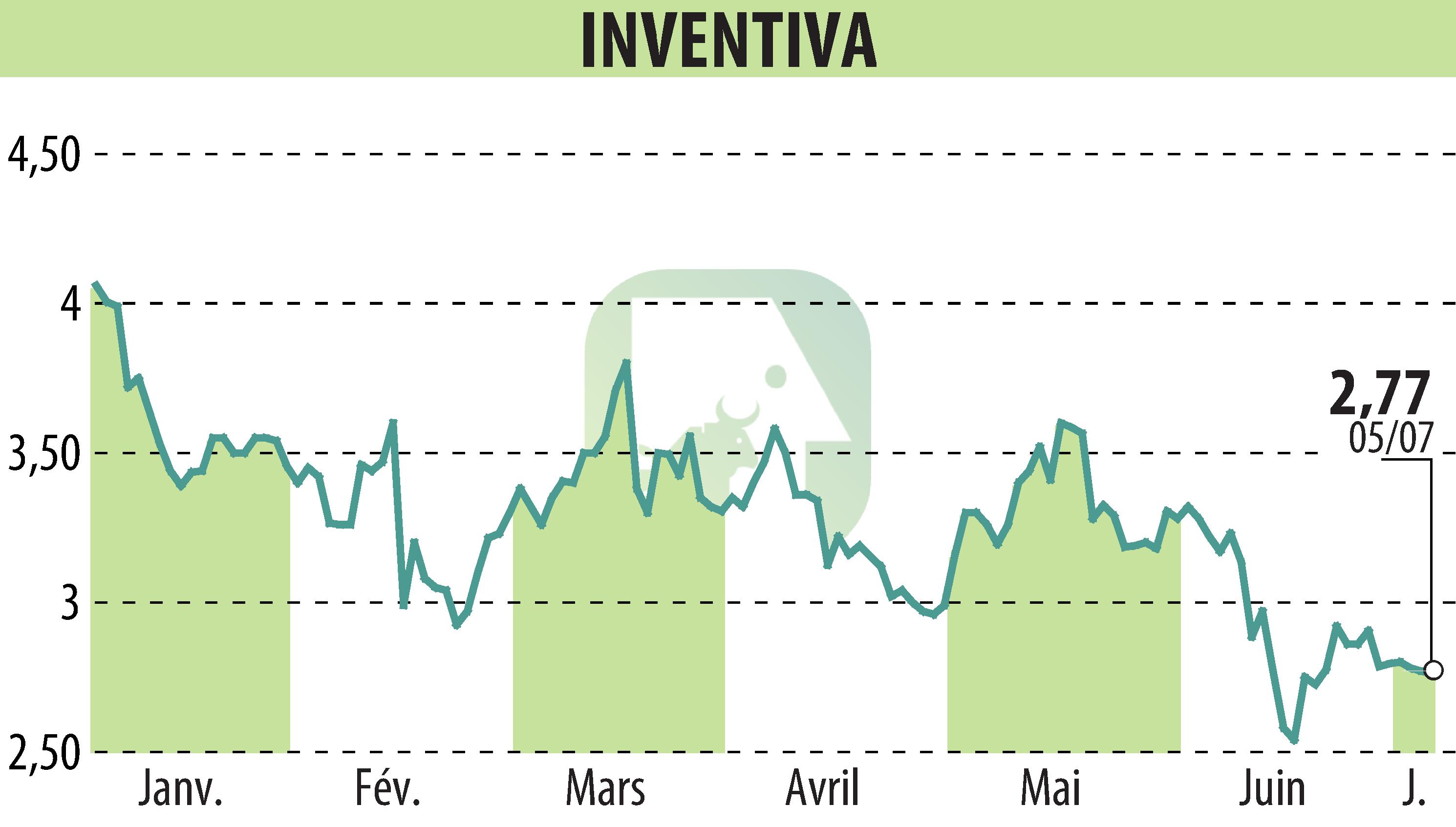
Inventiva announces significant progress in its NATiV3 phase III clinical trial. To date, more than 80% of patients planned for the main cohort have been recruited. For the exploratory cohort, the objective has already been achieved with 243 patients enrolled. The first visit of the last patient is planned for the second half of 2024, and the main results in 2026.
Analysis of baseline patient characteristics shows a profile similar to that of the phase IIb trial. The plateau effect observed in weight gain after 24 to 36 weeks is particularly encouraging for the safety of the treatment.
Inventiva is also strengthening its patent portfolio, with new protection for lanifibranor until 2043. The financial situation remains fragile. The company is exploring various financing options to continue operations beyond July 2024.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de INVENTIVA
