sur Jaguar Health, Inc. (NASDAQ:JAGX)
Investigator-Initiated Trial of Jaguar Health’s Crofelemer Yields Positive Results for Functional Diarrhea
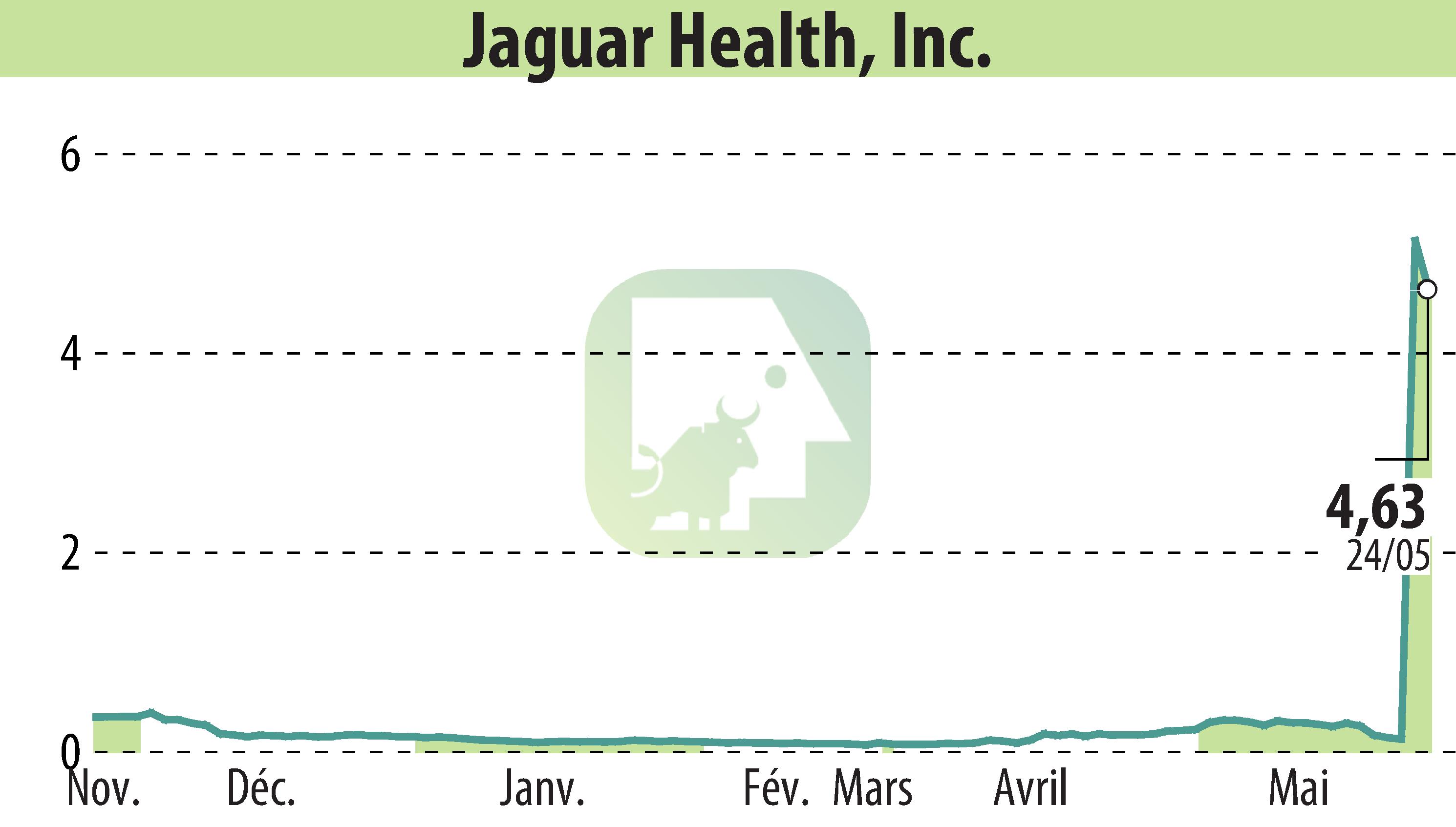
Jaguar Health, Inc., through its subsidiary Napo Pharmaceuticals, announced positive results from an investigator-initiated study of crofelemer, a plant-based prescription drug. Administered orally twice daily, crofelemer significantly reduced abdominal pain and discomfort, improving stool consistency in patients with functional diarrhea compared to a placebo.
The abstract of the study is set to be submitted to a peer-reviewed journal later this year. Functional diarrhea is defined as chronic diarrhea lasting more than six months and affects approximately 4.7% of people worldwide according to a Rome Foundation survey.
Crofelemer is derived from the red bark sap of the Croton lechleri tree, found in the Amazon Rainforest. Napo Pharmaceuticals has implemented a sustainable harvesting program under fair trade practices to ensure high-quality production and support Indigenous communities.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
