sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Announces Key Commercial and Development Milestones
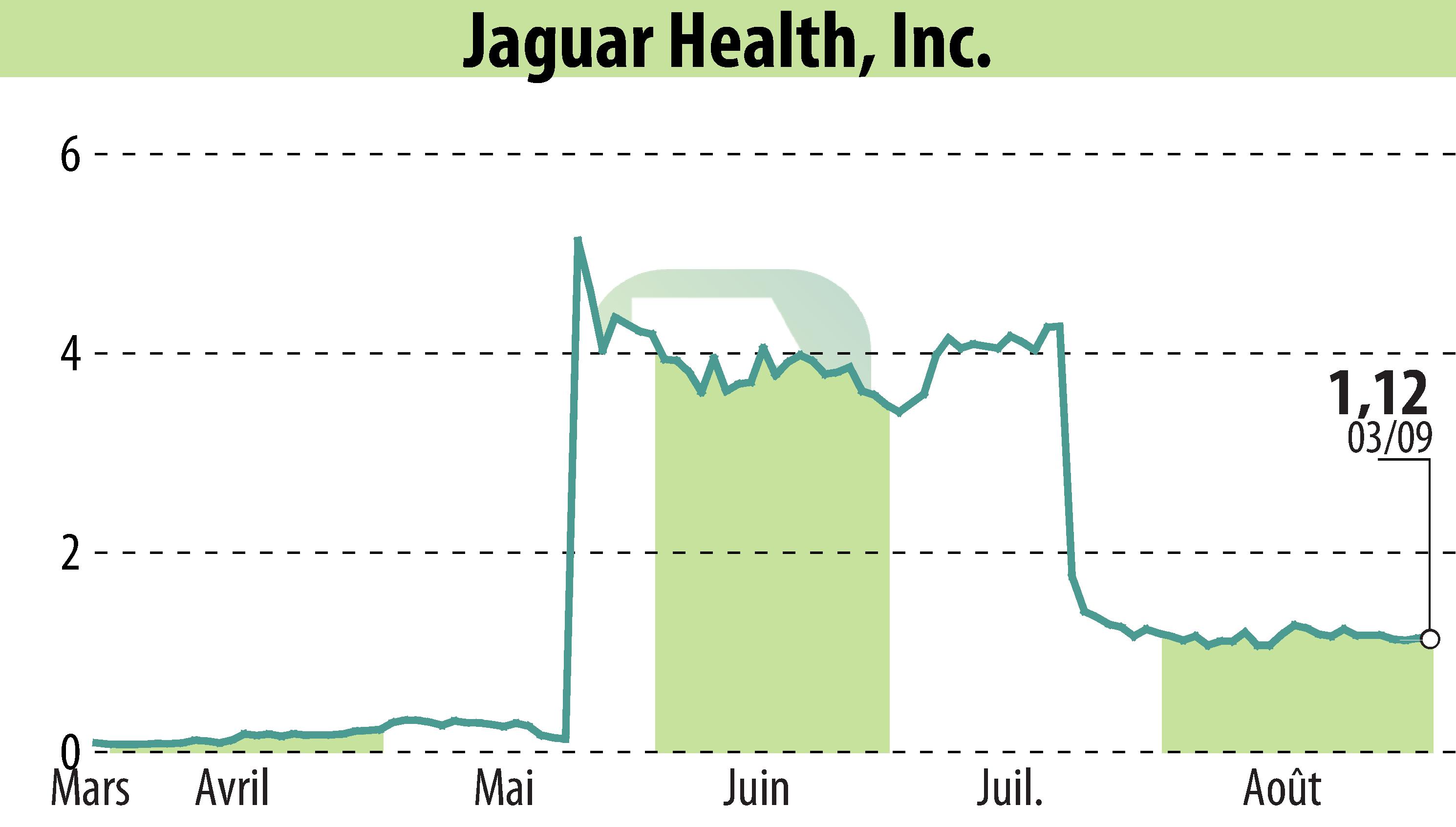
Jaguar Health, Inc. (NASDAQ:JAGX) has announced an upcoming October 2024 commercial launch of Gelclair®, an FDA-approved oral mucositis prescription product. The company also highlighted ongoing development activities for crofelemer, aimed at treating rare diseases such as microvillus inclusion disease (MVID) and short bowel syndrome with intestinal failure (SBS-IF).
In an interview with Jane King, CEO Lisa Conte discussed Jaguar Health's short-term goals and the expected results from several proof-of-concept studies by the end of 2024. Additionally, two global phase 2 studies for MVID and SBS-IF are set to enroll patients in Q4 2024, including a study in Abu Dhabi focusing on pediatric patients.
Conte noted that data from these studies could support early patient access to crofelemer in specific EU countries. Jaguar is also analyzing its phase 3 OnTarget trial for cancer therapy-related diarrhea, with plans to discuss potential FDA approval for crofelemer's use in breast and lung cancer patients.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
