sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Extends Collaboration for NP-300 Development
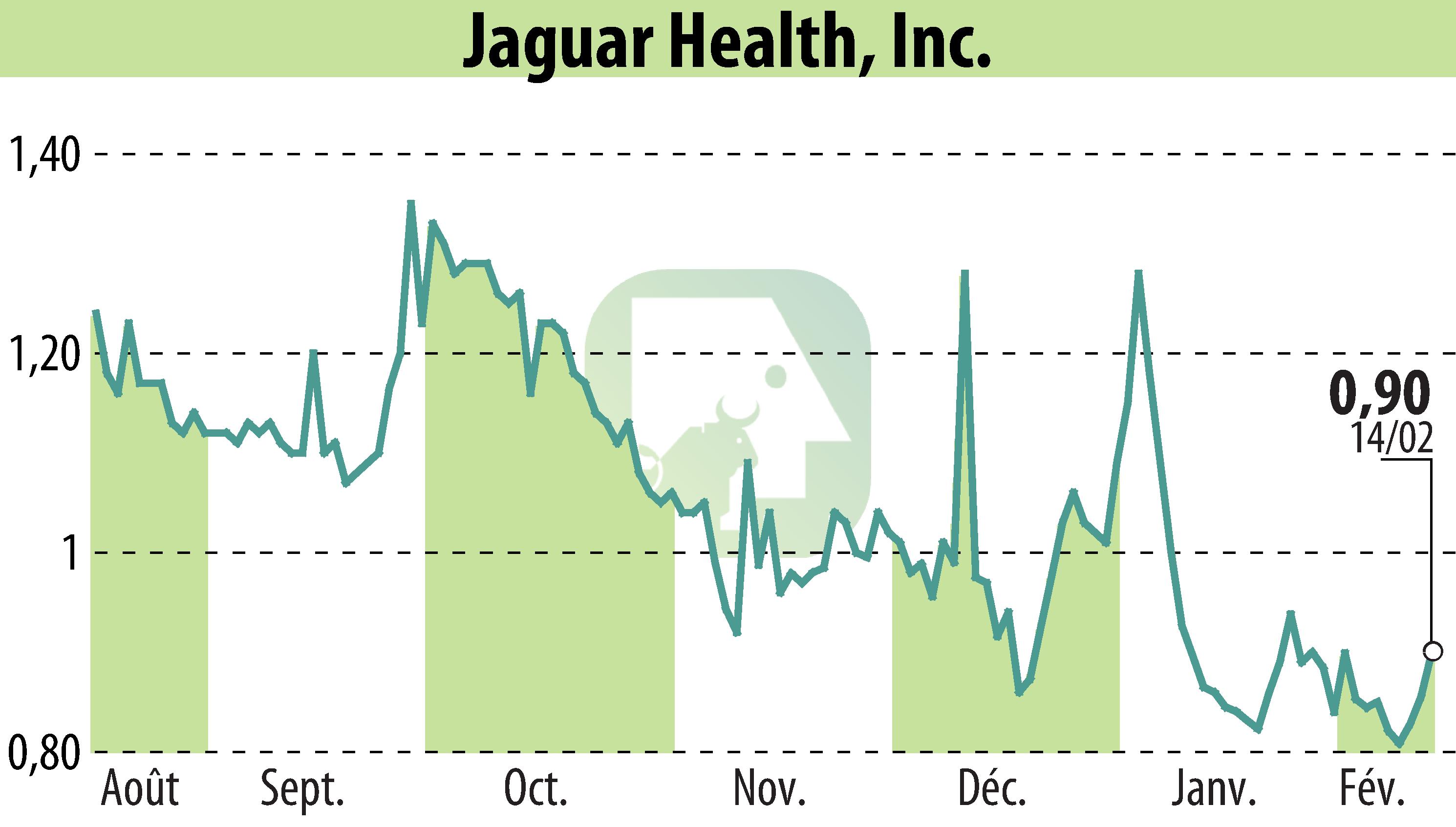
Jaguar Health, Inc. and Napo Pharmaceuticals have extended their partnership with Streeterville to advance the NP-300 drug candidate. This drug aims to alleviate symptoms of cholera, such as diarrhea and dehydration. They seek a Tropical Disease Priority Review Voucher (TDPRV) from the FDA, which could enhance market interest due to limited PRV availability.
Cholera impacts 1.3 to 4 million individuals globally each year, with 21,000 to 143,000 fatalities. The potential TDPRV could add significant value, given past voucher transactions reaching $350 million. The NP-300 project aligns with Jaguar's focus on treatments for neglected diseases.
Jaguar also anticipates further progress on other conditions, with proof-of-concept trial results expected throughout 2025 for conditions like short bowel syndrome. The strategic focus remains on leveraging botanical extraction methods for pharmaceutical advancements.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
