sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Files Patent for Crofelemer to Address GI Side Effects
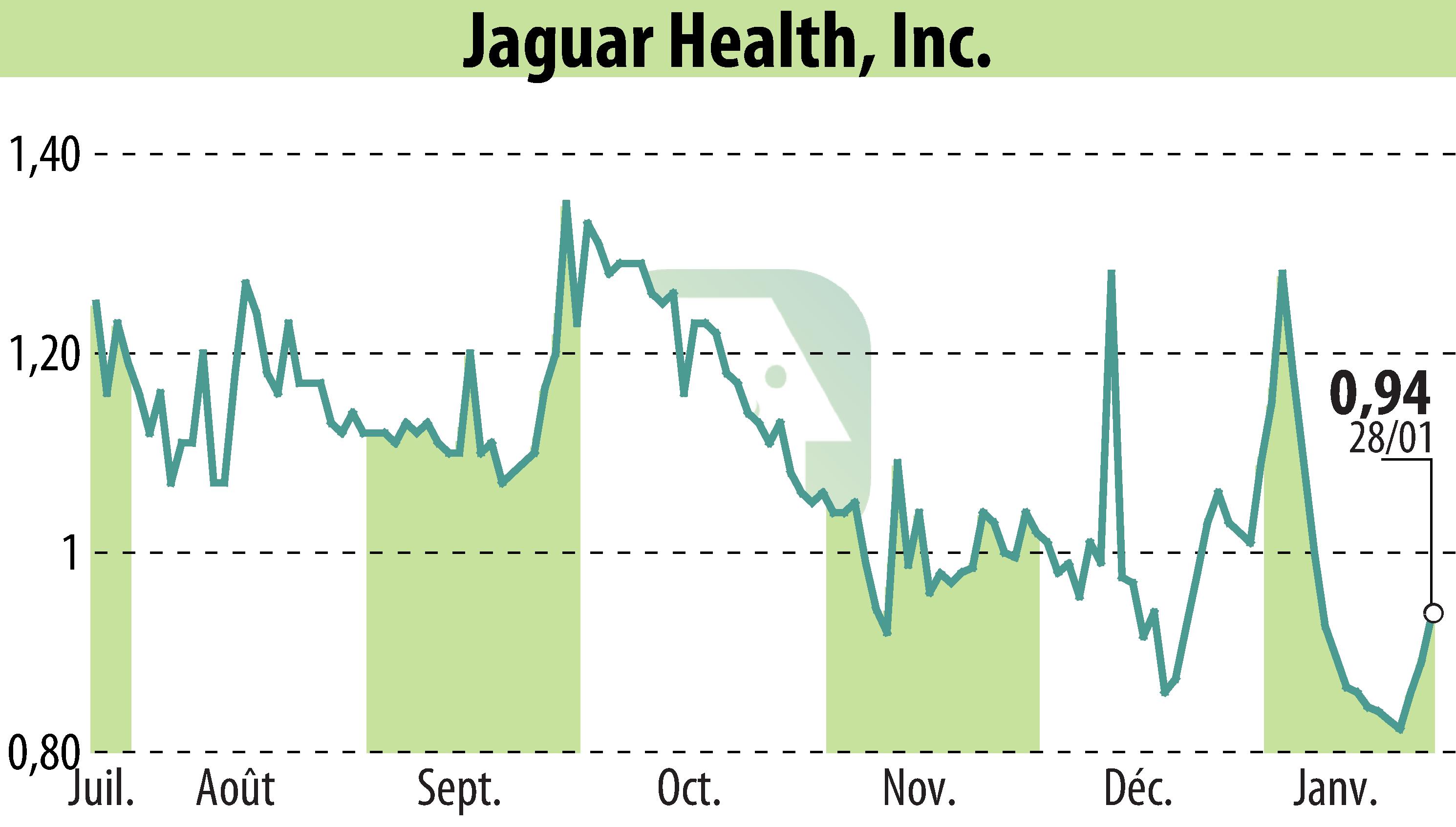
Jaguar Health, Inc. has announced a patent application for its FDA-approved drug crofelemer. This move seeks to address gastrointestinal (GI) side effects from GLP-1 receptor agonists, widely used in weight loss therapies. GI disorders are common with these drugs, affecting 40-70% of patients. In some trials, the rate was as high as 85%.
Crofelemer, branded as Mytesi®, is approved for HIV-related diarrhea. It shows promise in treating GI symptoms like diarrhea, abdominal pain, and constipation. The drug's potential extends to cancer therapy-related diarrhea and irritable bowel syndrome.
Jaguar Health's Napo Pharmaceuticals leads this initiative. They emphasize the global market's growth, with GLP-1 therapies projected to reach $322 billion by 2034. Crofelemer's role could be significant in helping patients continue their treatments comfortably.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
