sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks FDA Approval for Canine Diarrhea Treatment
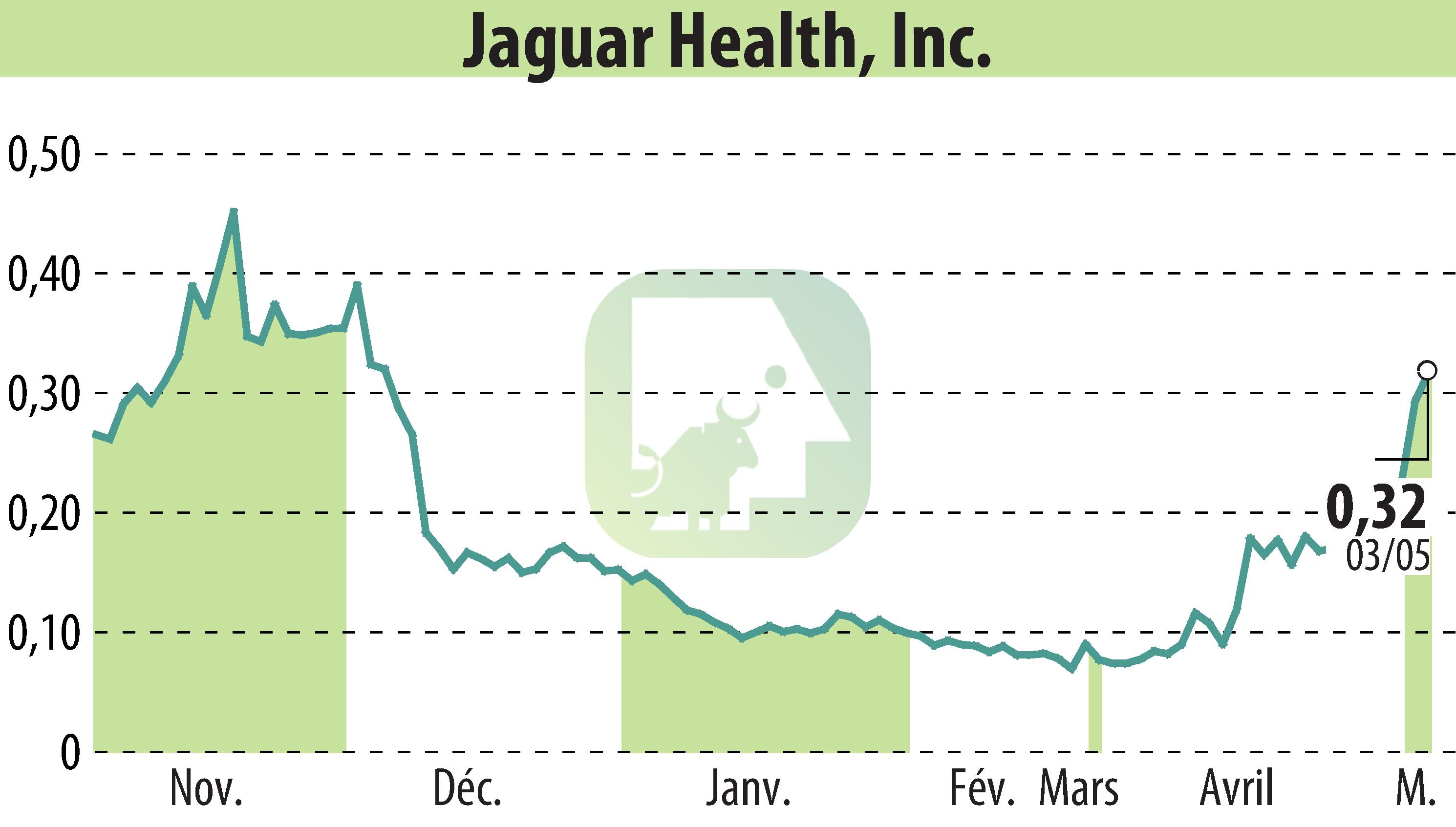
Jaguar Health, Inc. announced a new Investigational New Animal Drug (INAD) filing with the FDA's Center for Veterinary Medicine to potentially expand the use of its drug, crofelemer. Marketed as Canalevia®-CA1, it is currently conditionally approved for treating chemotherapy-induced diarrhea in dogs. The company aims to extend its application to general, non-infectious diarrhea, addressing an unmet need highlighted by the high incidence of this condition among dogs in the U.S.
Michael Guy, Vice President at Jaguar, expressed optimism about the market's reception of crofelemer and its future potential. The expansion targets approximately six million annual cases of canine diarrhea. Next steps include a pre-submission conference with the CVM to discuss clinical study protocols. Previously, crofelemer demonstrated efficacy in a controlled study, significantly outperforming a placebo in treating secretory diarrhea symptoms in dogs.
Jaguar highlighted the significant impact of diarrhea on dog health and the associated challenges faced by pet owners, particularly in urban areas. Canalevia-CA1 poses a remedy that could help manage this prevalent issue. However, it comes with specific handling instructions to avoid misuse, emphasizing its use under veterinary guidance.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
