sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks Partner for Dog Diarrhea Drug NP300
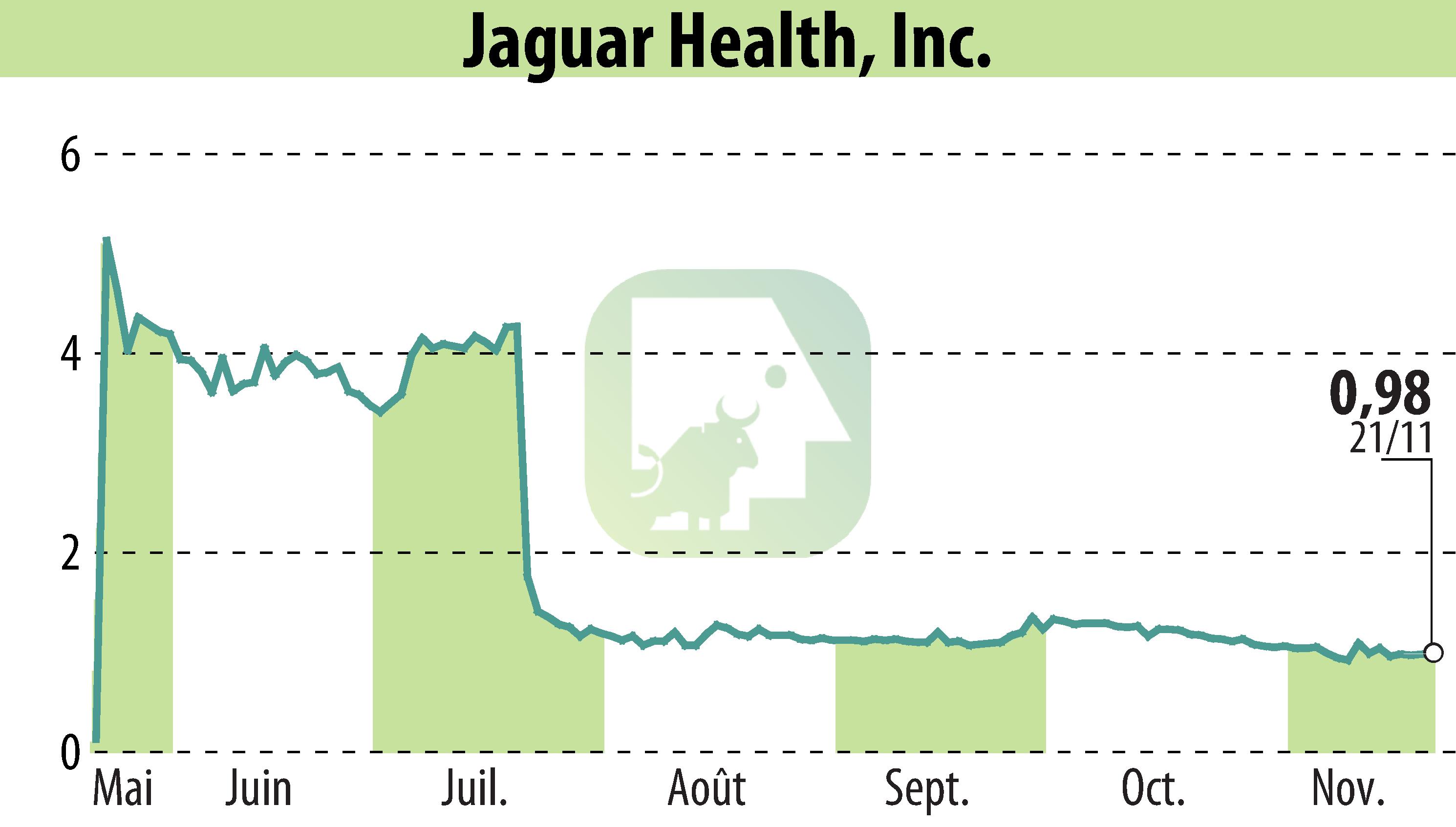
Jaguar Health, Inc. is in search of a partner to aid in the development and commercialization of NP300, a new drug candidate for treating general, non-infectious diarrhea in dogs. This move follows the success of their first-generation drug, Canalevia®-CA1, which targets chemotherapy-induced diarrhea in dogs and recently received extended conditional FDA approval.
The need for such a treatment is urgent, as there are currently no FDA-approved medications for general canine diarrhea—a condition frequently leading to veterinary visits. Jaguar plans to engage with potential partners at the December 3-5 Pet Connect conference in Hollywood, California. The company aims to grant commercial rights in the U.S. to a suitable partner to expedite NP300's market entry.
NP300 shares a mechanism with Canalevia®-CA1, utilizing crofelemer from the Croton lechleri tree, and has shown safety in trials. Given the prevalence of diarrhea amongst dogs and the lack of treatment options, Jaguar sees a significant unmet need in the market.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
