sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health's Crofelemer Shows Promise in Treating Cancer Therapy-Related Diarrhea
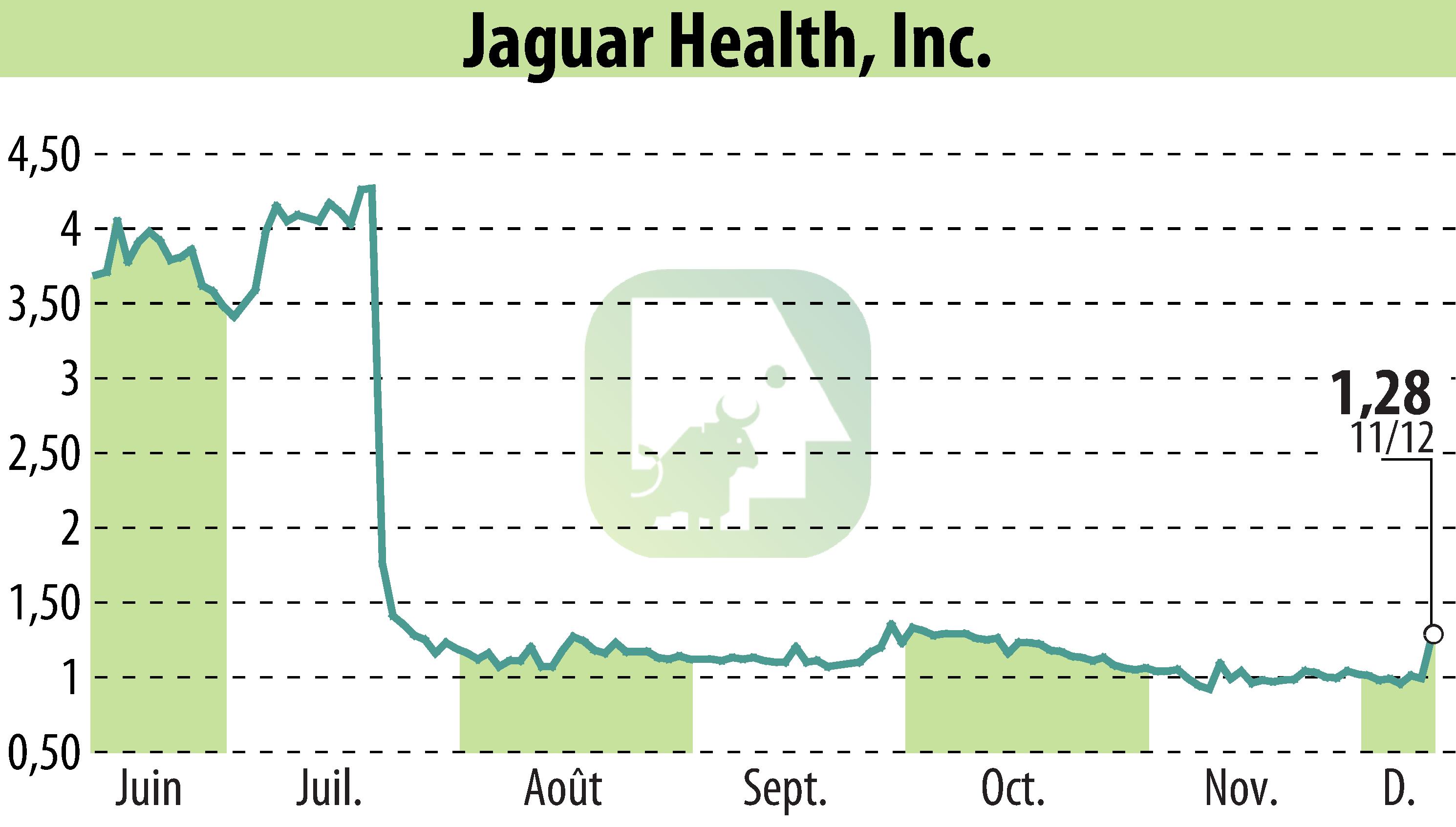
Jaguar Health has reported positive phase 3 trial outcomes for crofelemer in tackling cancer therapy-related diarrhea (CTD) among breast cancer patients. Findings were presented at the San Antonio Breast Cancer Symposium (SABCS). Crofelemer outperformed placebo in reducing diarrhea symptoms, enabling patients to maintain their cancer treatments. Significantly, 47.1% of crofelemer recipients were responders, compared to 33.7% in the placebo group. These results underscore crofelemer's potential in CTD management, crucial for treatment adherence and patient outcomes.
Diarrhea is common with therapies like abemaciclib and pertuzumab, often leading to dose adjustments. In the study, over 43% of abemaciclib users and all on pertuzumab required dose changes. Managing CTD could enhance patients' quality of life and treatment effectiveness. Jaguar Health plans to use these findings to engage with the FDA about crofelemer's potential pathway to availability for breast cancer patients. Further analyses of OnTarget trial data are expected to inform future submissions to scholarly forums.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
