sur MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Announces Final Phase Before IND Submission
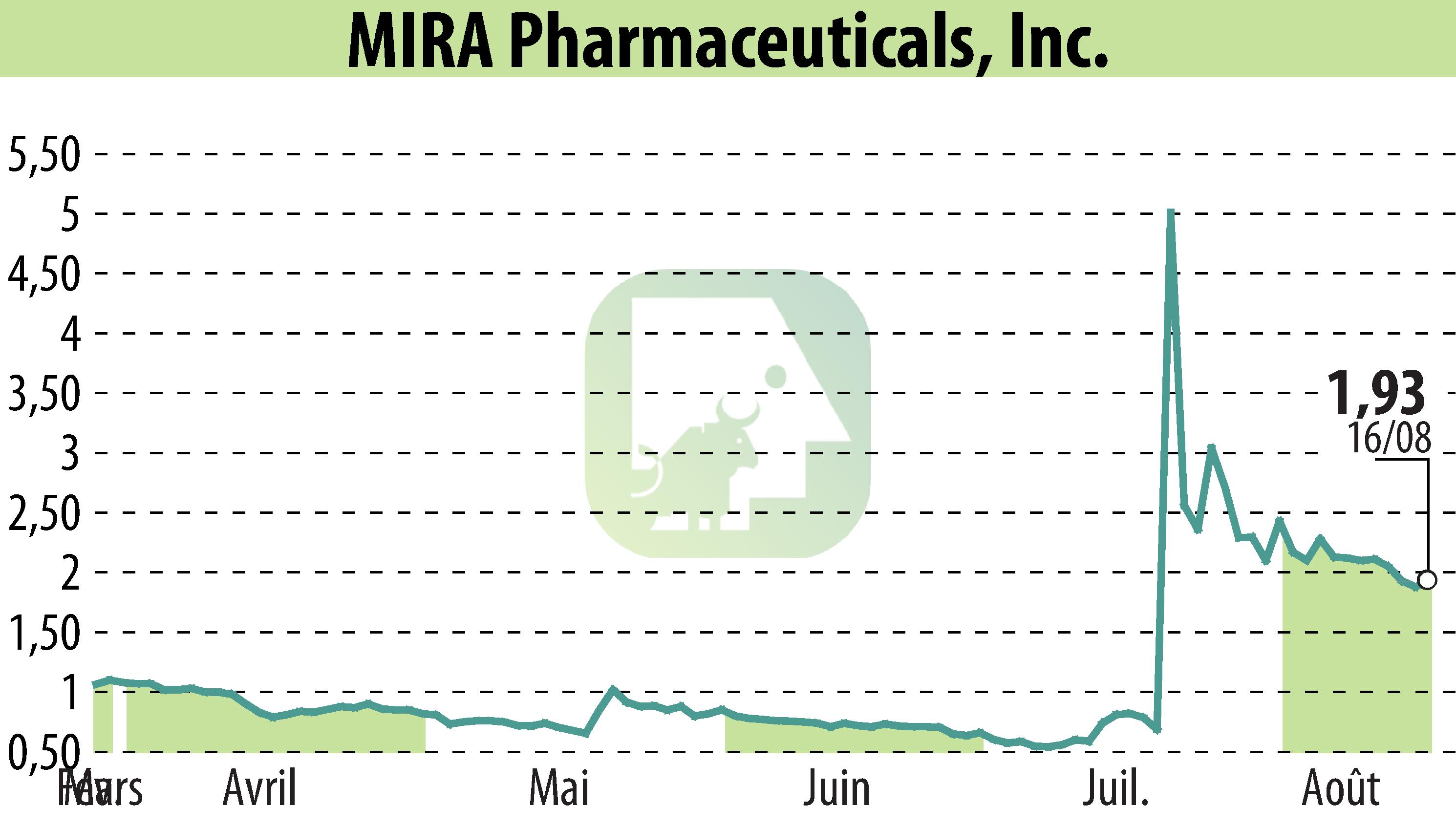
MIRA Pharmaceuticals Inc. (NASDAQ:MIRA) has announced significant progress as it prepares to submit its Investigational New Drug (IND) application by the end of the year. The company has made strategic advancements, particularly with its oral ketamine analog, Ketamir-2.
Leadership has been bolstered by Dr. Itzchak Angel, an expert in drug development, guiding MIRA’s innovative pipeline. Additionally, the company has highlighted Ketamir-2’s improved manufacturing process and safety profile, making it a potential breakthrough for mental health treatments.
Ketamir-2’s unique properties include enhanced oral absorption and reduced side effects. The compound’s key metabolite, Nor-Ketamir, shows promise for at-home neurological treatments due to its high oral bioavailability.
MIRA also announced progress with its oral marijuana analog, MIRA-55, which demonstrates higher efficacy and a consistent anxiolytic effect without being classified as a controlled substance by the DEA.
The company is finalizing GMP processes and regulatory safety studies for Ketamir-2, aiming for accelerated clinical trial pathways. This milestone reflects MIRA’s commitment to advancing neurological and neuropsychiatric disorder treatments.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MIRA Pharmaceuticals
