sur Moderna, Inc. (NASDAQ:MRNA)
Moderna Announces Positive Phase 3 Efficacy Data for mRNA-1283, the Company’s Next Generation COVID-19 Vaccine
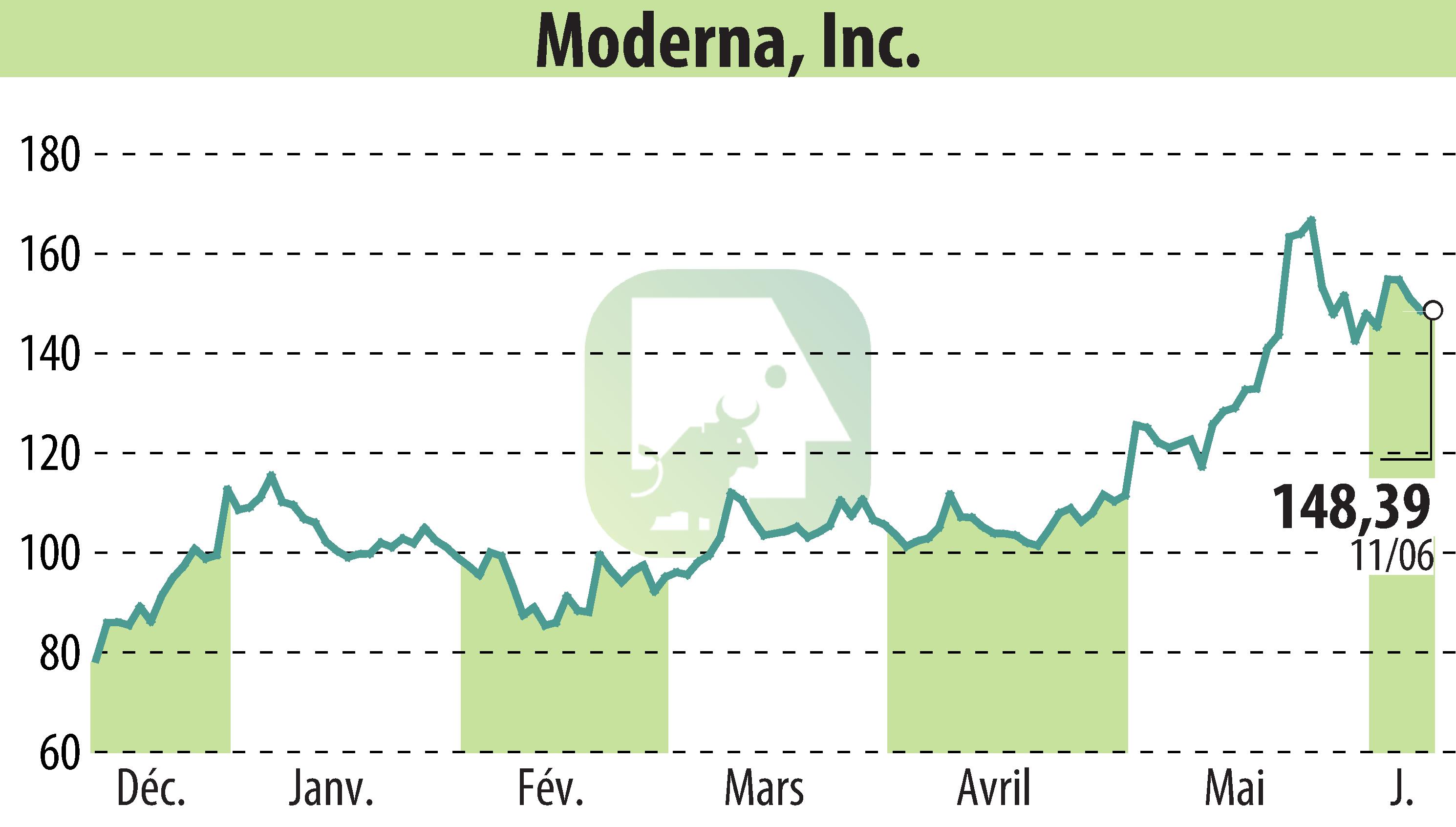
Moderna, Inc. (NASDAQ:MRNA) has announced that its Phase 3 trial of mRNA-1283, a next-generation COVID-19 vaccine, has met its primary efficacy endpoint. The trial showed that mRNA-1283 demonstrated non-inferior vaccine efficacy against COVID-19 compared to Spikevax® in participants aged 12 and older.
Notably, higher efficacy was observed in mRNA-1283 compared to Spikevax in adults aged 18 and older. A consistent trend was also seen in adults aged 65 and older. Positive interim immunogenicity results for mRNA-1283 were previously reported.
The study involved approximately 11,400 individuals, with half receiving a 10 μg dose of mRNA-1283 and the other half a 50 μg dose of mRNA-1273 (Spikevax). The vaccine efficacy data aligns with earlier findings showing higher neutralizing antibody responses against Omicron BA.4/5 and ancestral SARS-CoV-2 in mRNA-1283.
Moderna plans to present the Phase 3 clinical data at an upcoming conference and submit it for publication. The company will also engage with regulators for the next steps of the program.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
