sur Moderna, Inc. (NASDAQ:MRNA)
Moderna Doses First Participant in Phase 3 Trial of Norovirus Vaccine
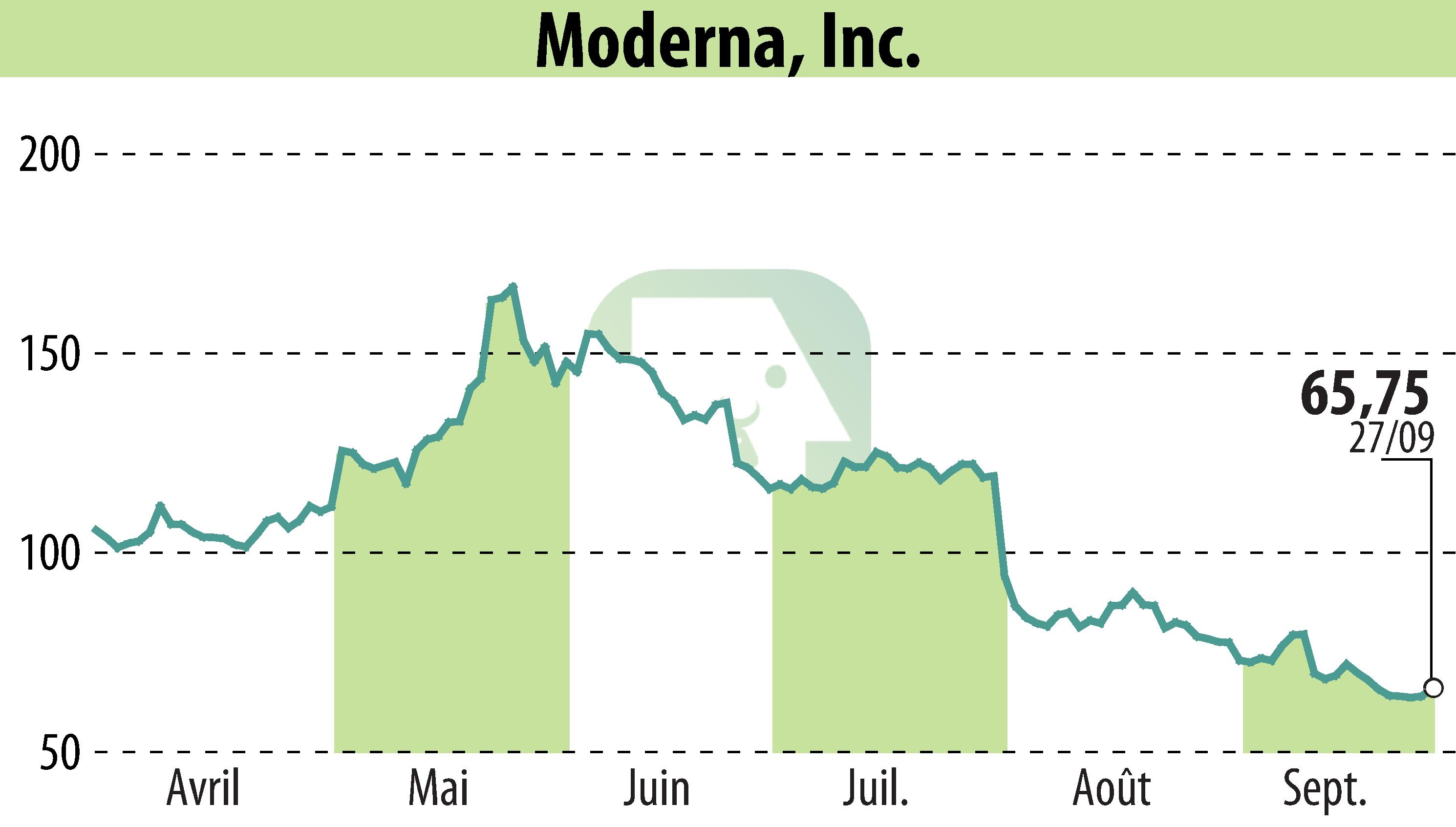
CAMBRIDGE, MA / ACCESSWIRE / September 30, 2024 / Moderna, Inc. (NASDAQ:MRNA) announced the commencement of the pivotal Nova 301 Phase 3 trial for its investigational norovirus vaccine, mRNA-1403. The first U.S. participant has been dosed, with global recruitment underway.
This randomized, observer-blind, placebo-controlled trial will assess the efficacy, safety, and immunogenicity of the mRNA-1403 vaccine. It aims to enroll 25,000 participants, focusing on adults aged 18 and older, with particular attention to those over 60 who are at higher risk for severe norovirus-related health outcomes.
Norovirus is a major public health issue, causing significant morbidity and mortality worldwide, especially among young children and older adults. Approximately 200,000 annual deaths are attributed to norovirus, underscoring the need for effective preventive measures.
mRNA-1403 is designed to protect against multiple norovirus genotypes. The successful delivery of this vaccine would be a critical tool in reducing the global burden of norovirus-related gastroenteritis.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
