sur Moderna, Inc. (NASDAQ:MRNA)
Moderna Files FDA Application for JN.1 Targeting COVID-19 Vaccine
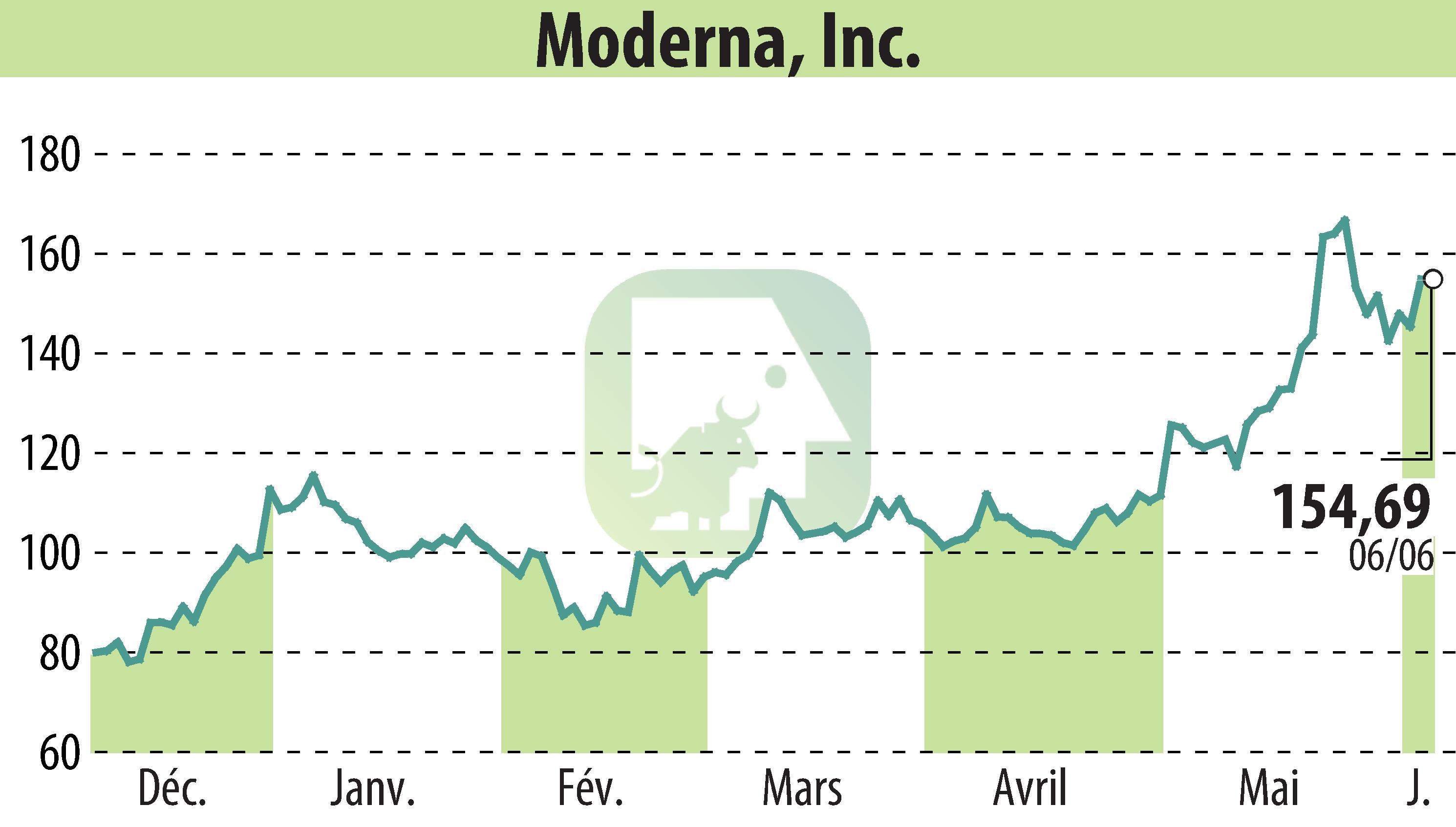
CAMBRIDGE, MA / ACCESSWIRE / June 7, 2024 / Moderna, Inc. (NASDAQ:MRNA) has taken a significant step in the fight against COVID-19. The company announced it has submitted an application to the U.S. Food and Drug Administration (FDA) for its Spikevax 2024-2025 formula. This new version of the vaccine targets the SARS-CoV-2 variant JN.1. Manufacturing is in progress, and doses will be ready for shipping as early as August, pending regulatory approval.
The submission follows FDA guidance that COVID-19 vaccines for the 2024-2025 season should be updated to a monovalent JN.1 composition. This aligns with recommendations from the World Health Organization (WHO) and the European Medicines Agency (EMA). Most solicited local adverse events reported include injection site pain, while systemic adverse events include headache, fatigue, myalgia, and chills.
Moderna’s CEO, Stéphane Bancel, emphasized the importance of staying current with COVID-19 vaccinations to protect against respiratory illnesses. The company is also submitting data to regulators worldwide to facilitate the registration and supply of its updated Spikevax formula in time for the next vaccination season.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
