sur Moderna, Inc. (NASDAQ:MRNA)
Moderna R&D Day Highlights Progress and Strategic Priorities
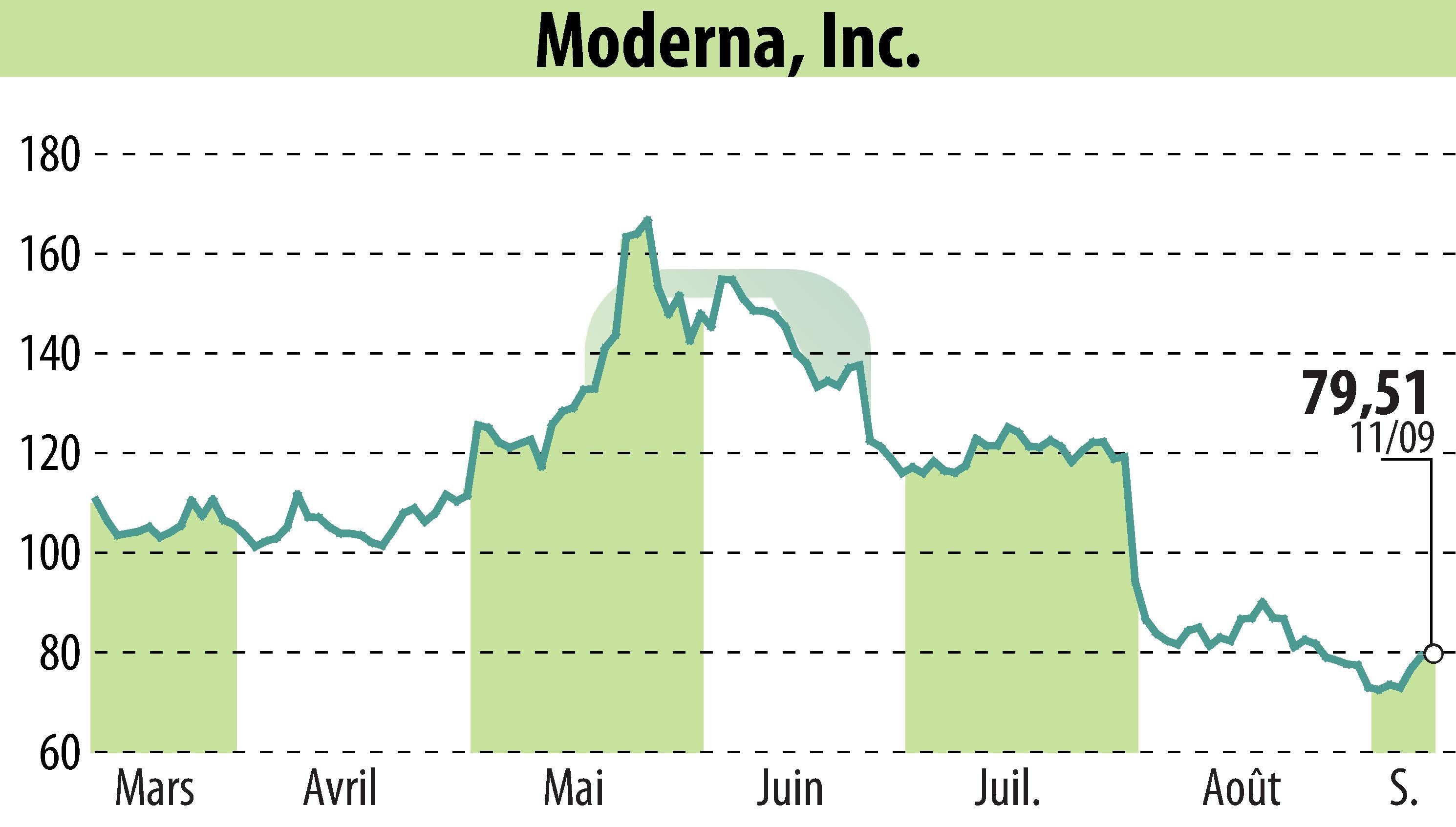
Moderna, Inc. (NASDAQ:MRNA) announced key updates at its annual R&D Day, focusing on strategic priorities and progress within its mRNA pipeline. The company aims for ten product approvals by 2027, including a next-generation COVID vaccine and a flu/COVID combination vaccine, both expected to be submitted for approval in 2024.
Moderna highlighted positive Phase 3 results for its RSV vaccine for high-risk adults aged 18 to 59 and its standalone flu vaccine for adults aged 65 and older. Additionally, the norovirus vaccine has advanced into Phase 3 trials, expanding the non-respiratory pivotal stage programs to five, covering oncology, rare diseases, and first-in-class vaccines.
To streamline costs, Moderna plans to reduce R&D expenses by $1.1 billion, from $4.8 billion in 2024 to $3.6-3.8 billion by 2027. The company also updated its financial framework through 2028, aiming to break even on an operating cash cost basis by that year.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
