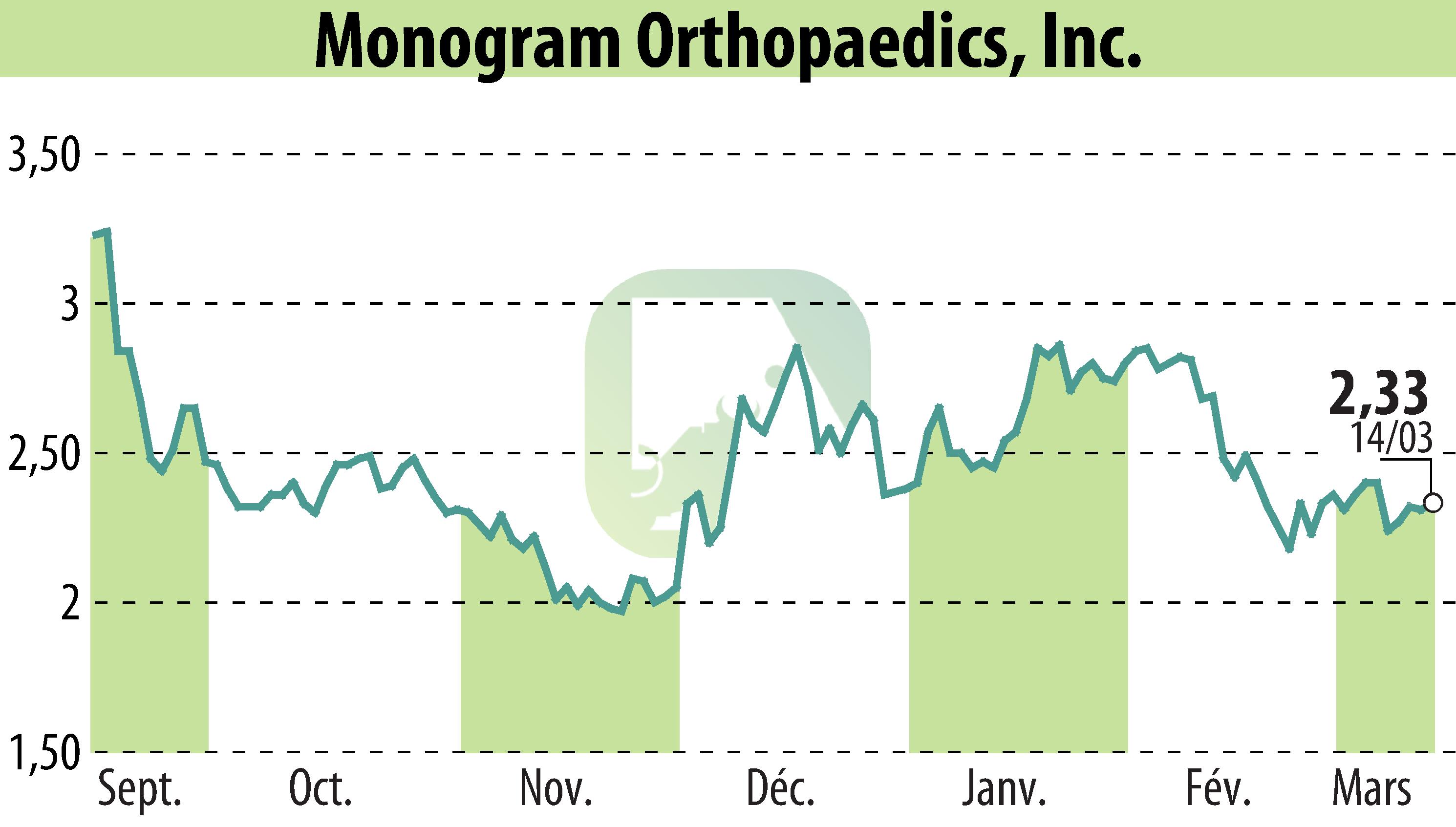
sur MONOGRAM ORTHOPAEDICS INC (NASDAQ:MGRM)
Monogram Technologies Secures FDA Clearance for mBôs TKA System
Monogram Technologies Inc. has received FDA 510(k) clearance for its Monogram mBôs™ TKA System. This clearance marks a significant milestone for the AI-driven robotics company, enabling the commercialization of its robotic-assisted technology for orthopedic surgery. The Monogram mBôs TKA System is developed to enhance safety, efficiency, and accuracy in total knee arthroplasty. The system is designed with scalability to support multiple orthopedic applications.
Ben Sexson, CEO of Monogram, emphasized the team's dedication in achieving this milestone and the potential for market impact. The company is focused on refining its strategy to support broader adoption and plans initial placements with key surgeons in strategic areas. Despite a highly consolidated market, the company aims to leverage its innovative technology for growth.
Monogram plans to integrate upgrades and system enhancements to improve competitiveness. The company is also exploring international opportunities, including potential clinical trials in collaboration with Shalby Limited in India. Its long-term strategy emphasizes gradual market adoption and technological refinement to expand the system's capabilities.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MONOGRAM ORTHOPAEDICS INC
