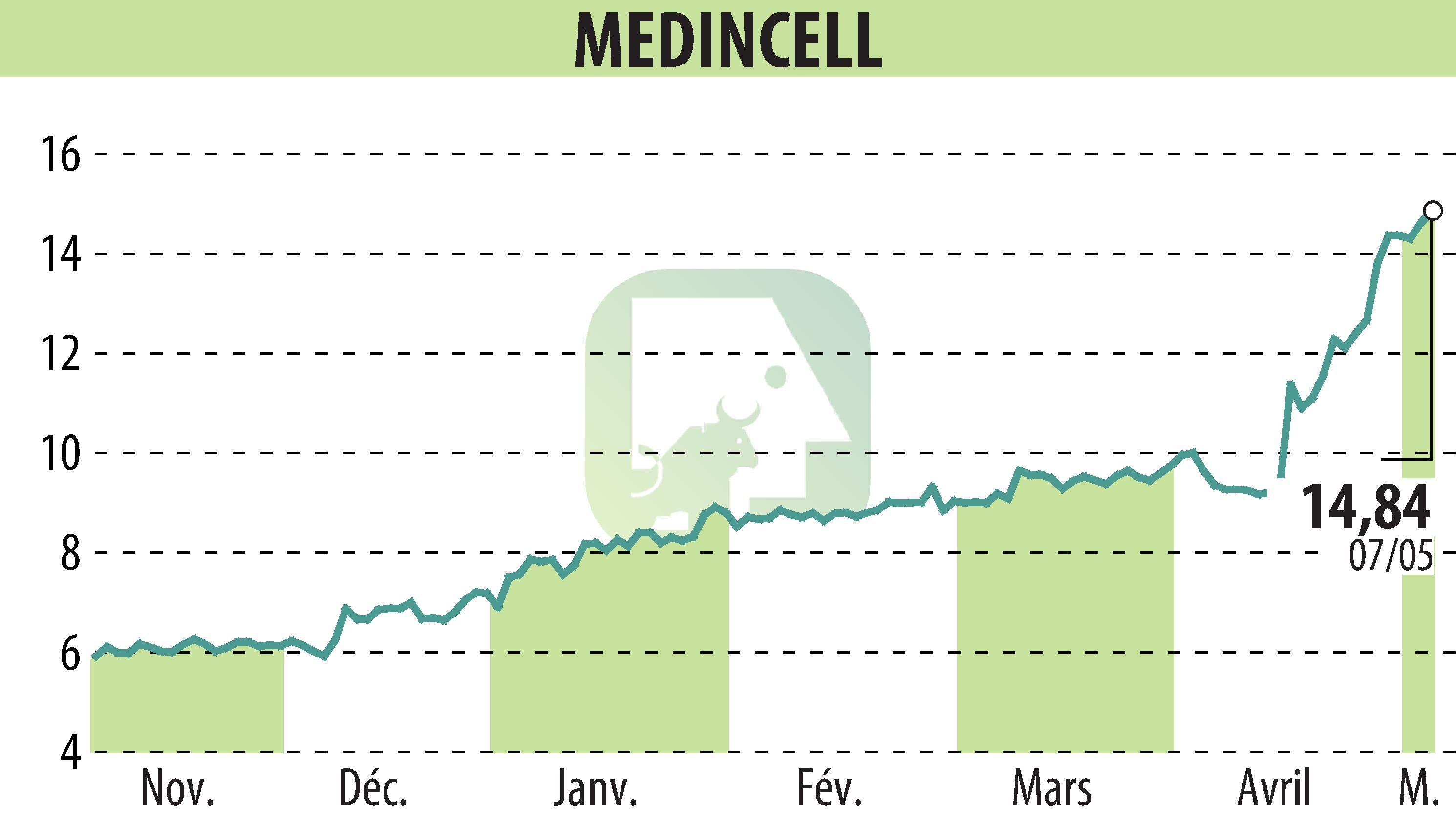
sur MEDINCELL (EPA:MEDCL)
Positive Results for the SOLARIS Phase 3 Trial of Olanzapine
The Teva and Medincell companies reported positive efficacy results for the phase 3 SOLARIS trial, evaluating the antipsychotic TEV-'749 (olanzapine/mdc-TJK) in adults with schizophrenia. The study met its primary endpoint by achieving significant reductions in PANSS scores, measuring the severity of schizophrenia symptoms, compared to placebo.
The drug TEV-'749 is well tolerated, showing no signs of post-injection delirium/sedation syndrome (PDSS). Medincell's SteadyTeq™ technology, which provides controlled release of the widely prescribed antipsychotic olanzapine, was used in the development of this injectable formulation. Preliminary results also indicate significant improvement on other secondary endpoints, with no occurrence of PDSS reported to date.
These encouraging results confirm the potential of TEV-'749 as a long-acting treatment, providing a new option for the management of schizophrenia without the risks associated with intramuscular olanzapine. Further efficacy and safety results are expected at future conferences, with continued monitoring planned in the second phase of the SOLARIS study.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MEDINCELL
