sur SENSORION (EPA:ALSEN)
Sensorion Completes Recruitment for NOTOXIS Trial of SENS-401
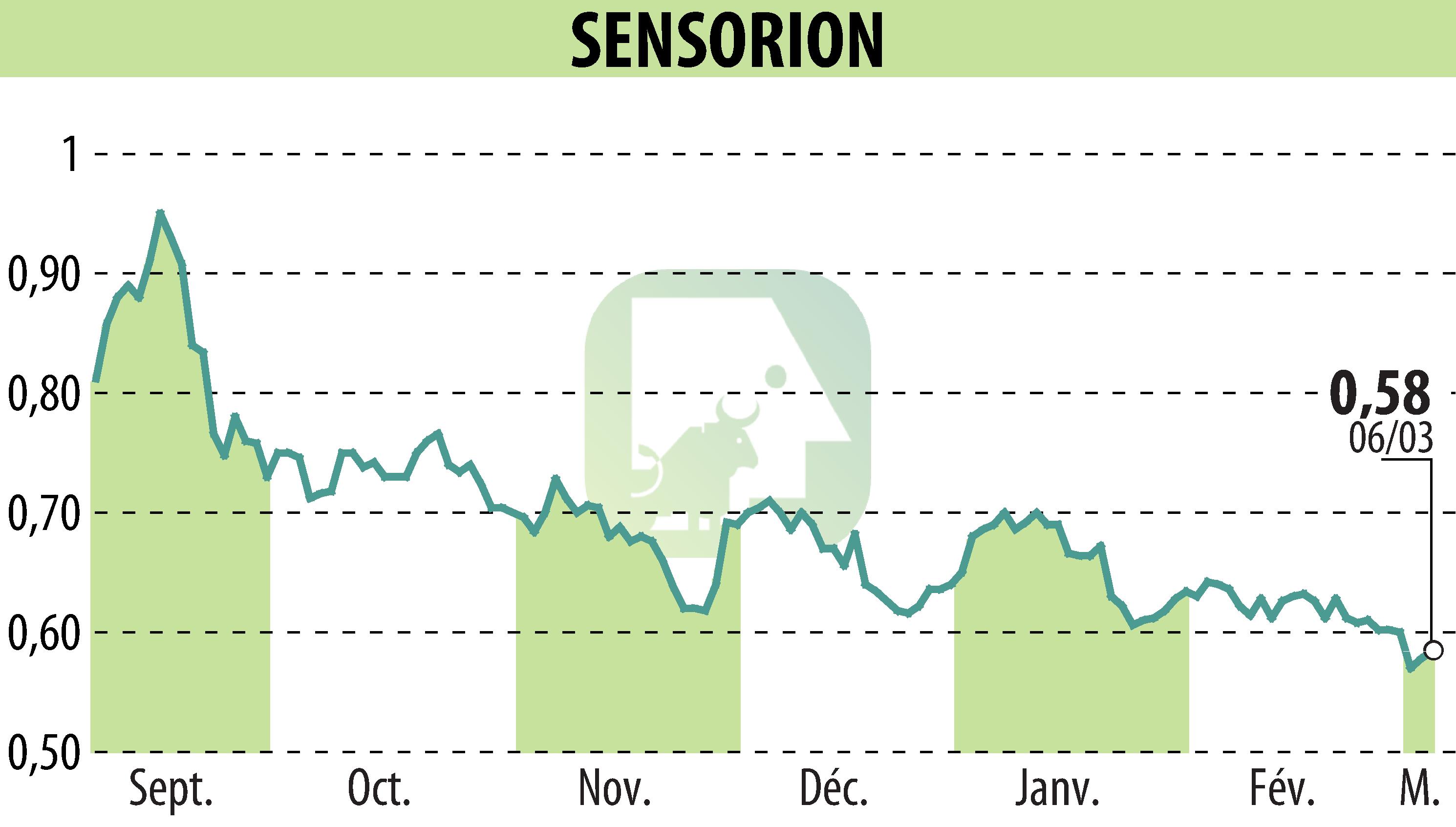
Sensorion announced that it has completed patient enrollment in its Phase 2a clinical trial of SENS-401. This program, called NOTOXIS, is designed to prevent cisplatin-induced ototoxicity, a severe side effect of certain chemotherapy treatments.
The trial includes 48 adult patients equally divided between a group receiving SENS-401 and a control group. Treatment involves daily administration of 43.5 mg of SENS-401 orally, over an extended period, including phases before, during and after chemotherapy.
Preliminary results, presented in September 2024, suggest efficacy of SENS-401 with a good safety profile. Full study results are expected by the end of 2025.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SENSORION
