sur SENSORION (EPA:ALSEN)
Sensorion Reports Positive Preliminary Results for SENS-501
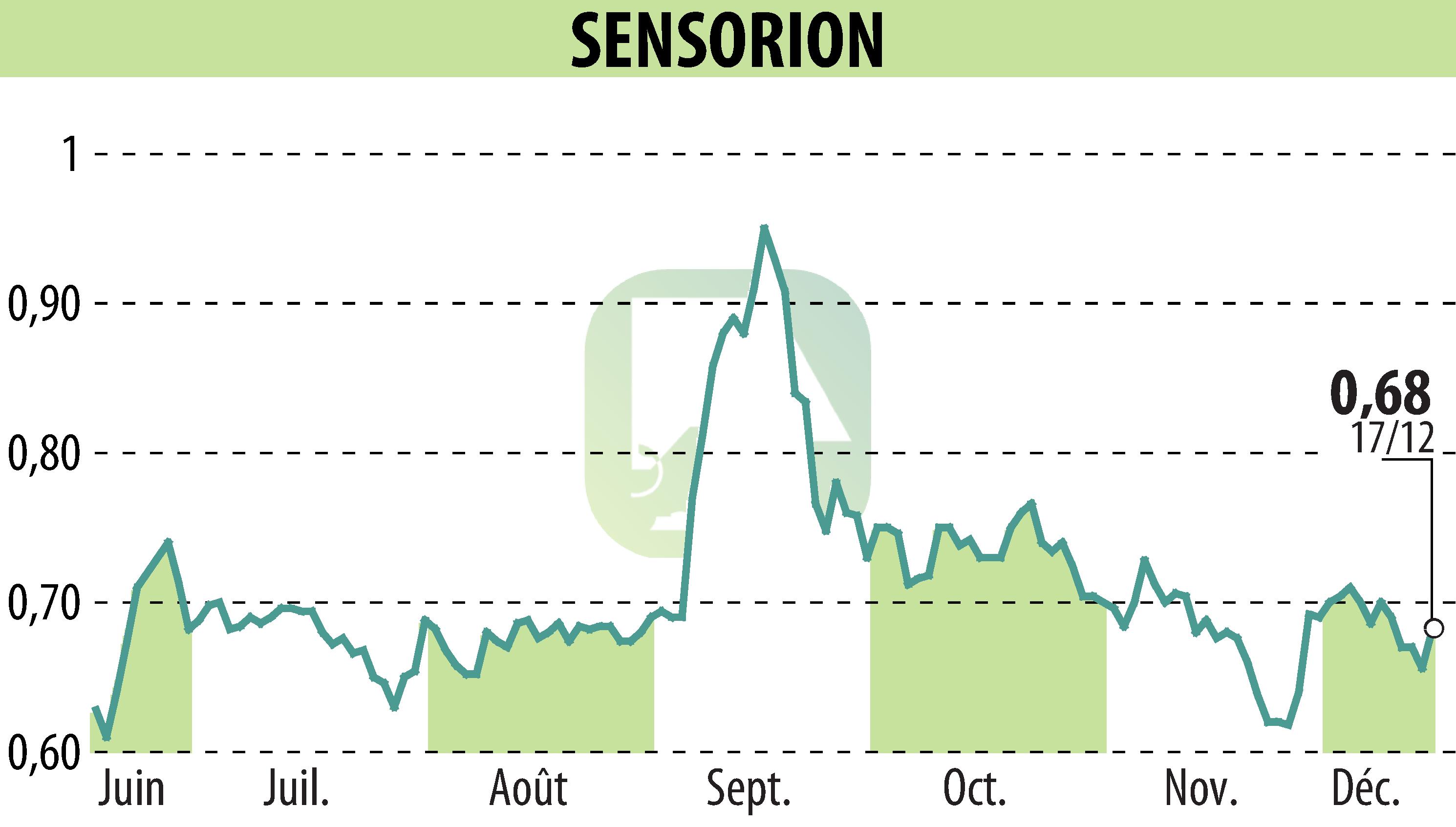
Biotech company Sensorion has revealed promising data from its Phase 1/2 Audiogene clinical trial focusing on the gene therapy SENS-501. This treatment is intended for infants suffering from congenital deafness due to mutations in the OTOF gene. The first two patients tolerated the treatment well without major incidents, and encouraging behavioral improvements were observed.
Sensorion plans to hold an online meeting in early 2025 to discuss the results and consider next steps in development, including discussions with the FDA. The company plans to enroll its second cohort by mid-2025.
Géraldine Honnet, Medical Director, expressed her satisfaction with the progress of the trial, promising new standards for treating otoferlin deficiency. Professor Natalie Loundon highlighted the potential of this therapy to improve hearing and quality of life for affected children.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SENSORION
