sur SENSORION (EPA:ALSEN)
Sensorion's SENS-501 Clinical Trial Progresses with Positive DMC Recommendation
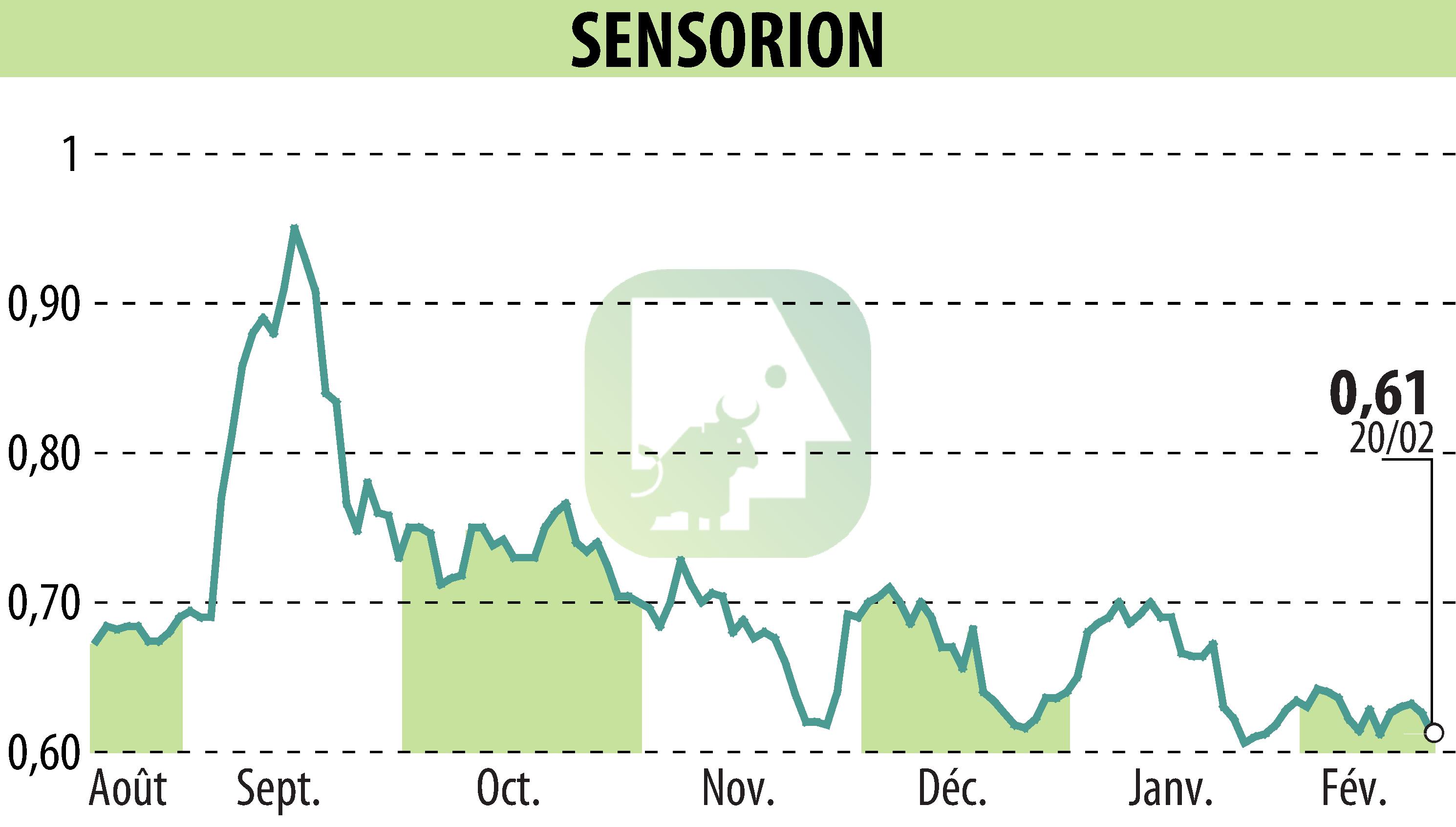
Sensorion, a clinical-stage biotech company focused on hearing loss therapies, announced a positive recommendation from the Data Monitoring Committee (DMC) for its Audiogene Phase 1/2 trial of SENS-501. This gene therapy targets congenital deafness related to OTOF gene mutations. The DMC's review affirmed the safety of the first dose level in young children, leading to the trial's continuation without changes.
The first cohort's recruitment of three patients, children aged 6 to 31 months, was completed in December 2024. Recruitment for the second cohort is on track to conclude by mid-2025. The Audiogene trial aims to determine the safety and efficacy of SENS-501 through intra-cochlear injections, potentially improving speech and language acquisition in children with pre-linguistic hearing loss.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SENSORION
