sur MEDINCELL (EPA:MEDCL)
Teva and Medincell Present Promising Results on Olanzapine LAI for Schizophrenia
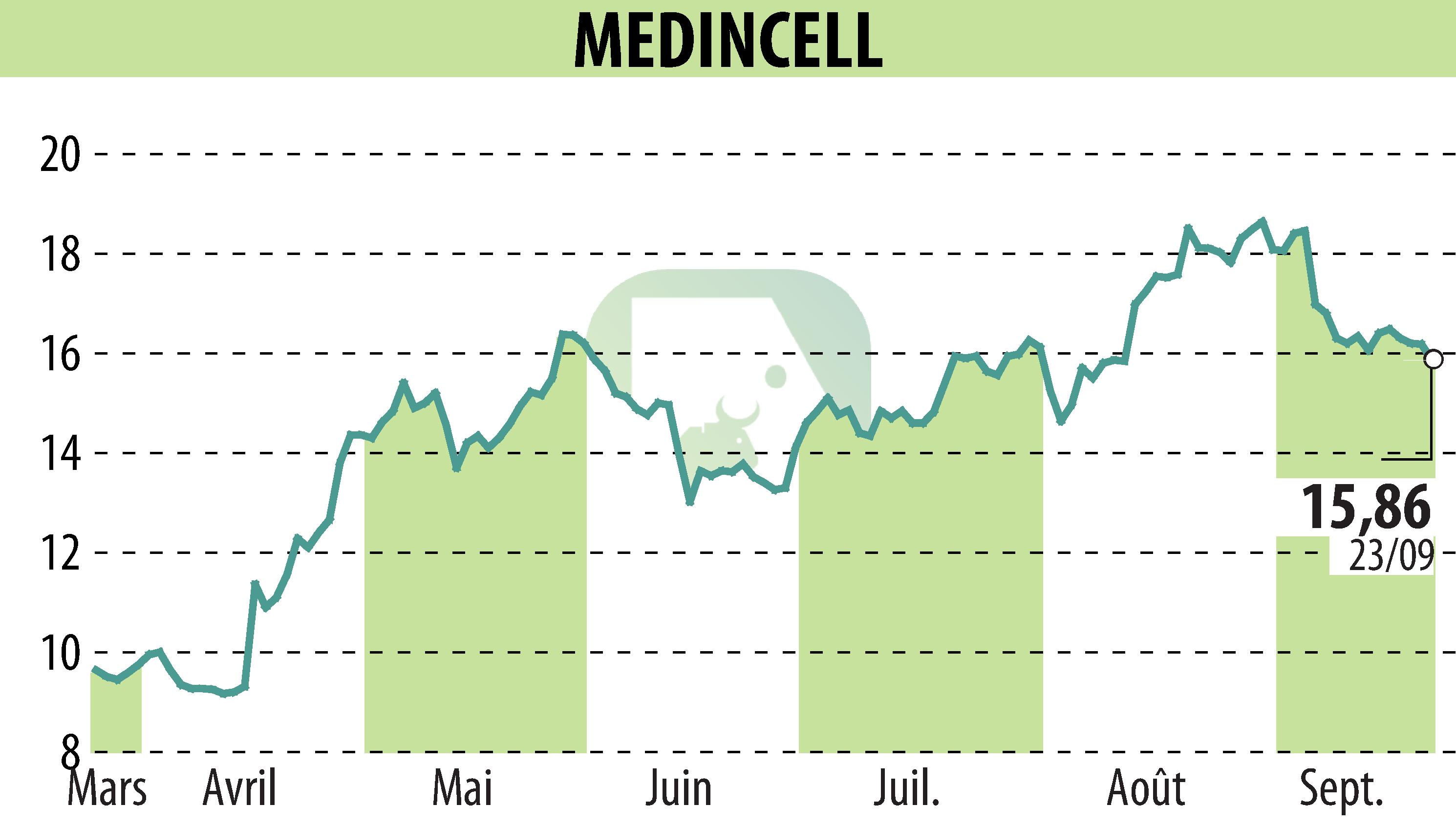
Montpellier, France - Teva, in partnership with Medincell, announced at ENCP 2024 positive results regarding the efficacy, safety and tolerability of the Phase 3 SOLARIS trial of Olanzapine LAI for adult patients with schizophrenia.
The 8-week clinical trial showed that all three tested doses of Olanzapine LAI met their primary and secondary efficacy endpoints, with significant improvements in PANSS, ICG-S and PSP scores compared to placebo. No cases of post-injection sedation syndrome (PDSS) were observed.
Richard Malamut, Medical Director of Medincell, stressed that this new injectable form could facilitate the transition of patients and improve the treatment of schizophrenia thanks to its favorable safety profile.
Teva, which is responsible for the development and commercialization of Olanzapine LAI, could pay Medincell up to $117 million in milestones and royalties in the coming years.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MEDINCELL
