sur Tharimmune Inc. (NASDAQ:THAR)
Tharimmune Shares Promising TH104 Data at Liver Meeting
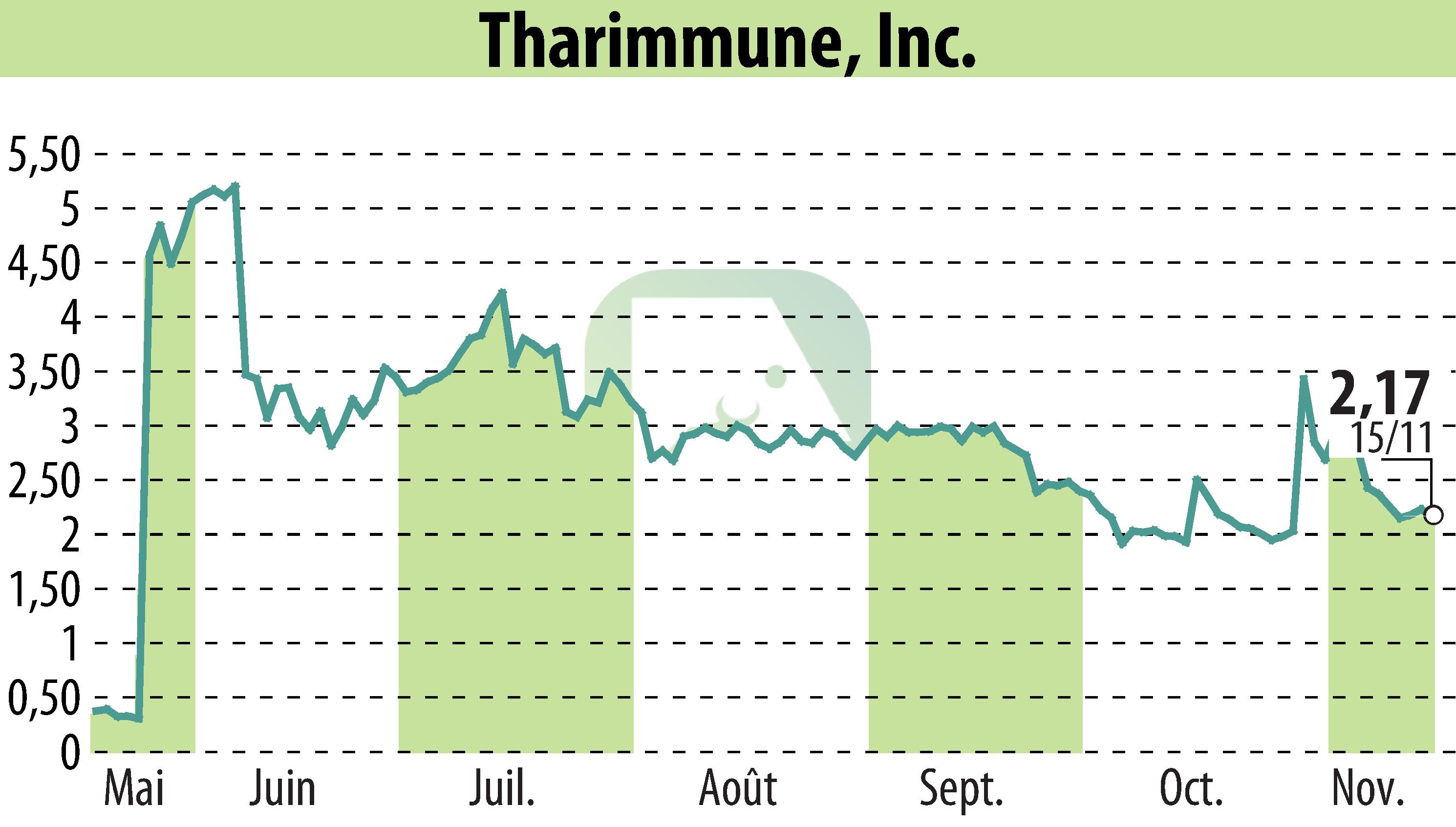
Tharimmune Inc., a clinical-stage biotechnology company, recently unveiled Phase 1 clinical data for its lead therapeutic, TH104, at the American Association for the Study of Liver Disease 2024 meeting in San Diego. The study focused on patients with chronic liver disease (CLD) and showed significant correlation between blood levels and symptom relief, with no unexpected adverse events observed.
The trial explored TH104's safety and efficacy in two cohorts categorized by Child-Pugh scores, assessing pruritus intensity using the Worst-Itch Numerical Rating Scale (WI-NRS). Notably, after administering a single low dose, participants experienced a notable reduction in WI-NRS scores, indicating relief from itch symptoms.
Tharimmune plans to advance to Phase 2 trials, targeting chronic pruritus in primary biliary cholangitis patients, with expectations to release preliminary data in 2025. CEO Randy Milby emphasized ongoing collaboration with U.S. and EU regulatory authorities for future development.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Tharimmune Inc.
