sur THERACLION (EPA:ALTHE)
Theraclion presents its achievements for the first half of 2024
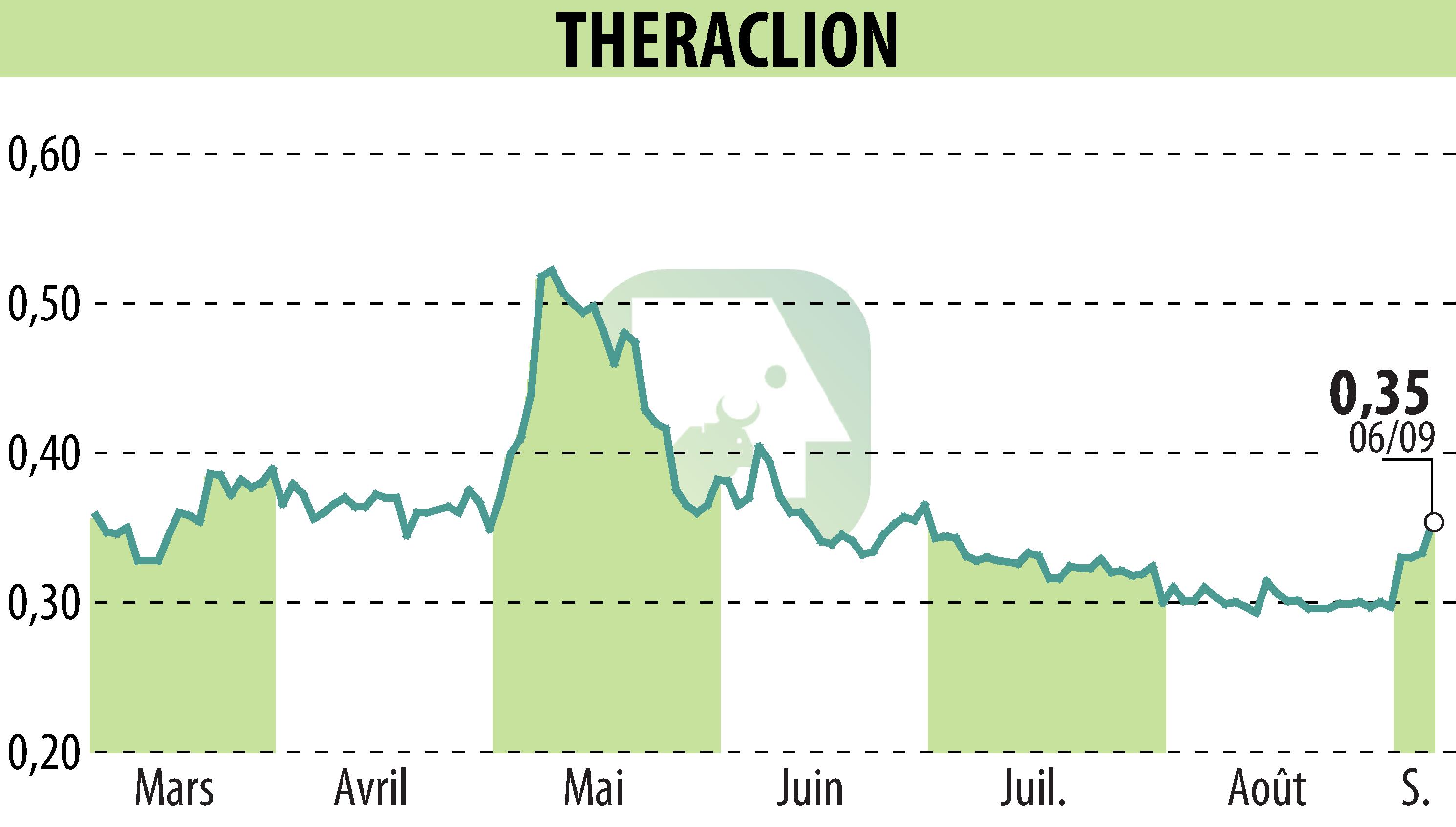
Theraclion, a specialist in non-invasive high-intensity focused ultrasound (HIFU) therapy, is reporting its progress for the first half of 2024. The company is highlighting a series of successes, particularly in the American and Chinese markets.
In June, Theraclion completed the US FDA pivotal study for the SONOVEIN® device. 70 patients were treated in four centers in Europe and the United States, marking a key milestone towards approval expected in early 2026.
Significant progress has been made in R&D, particularly on the processing speed of SONOVEIN®. These developments remain confidential to protect intellectual property. In addition, SONOVEIN® has increased its scientific visibility, with a success rate of 98.3% over 12 months.
In China, Theraclion made progress thanks to its joint venture with Furui, marked by the creation of an experienced team and the production of a first prototype. However, revenues fell by 82% due to a refocusing on existing customers and clinical trials.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de THERACLION
