sur Theranexus (EPA:ALTHX)
Theranexus presents positive results for the treatment of Batten disease CLN3
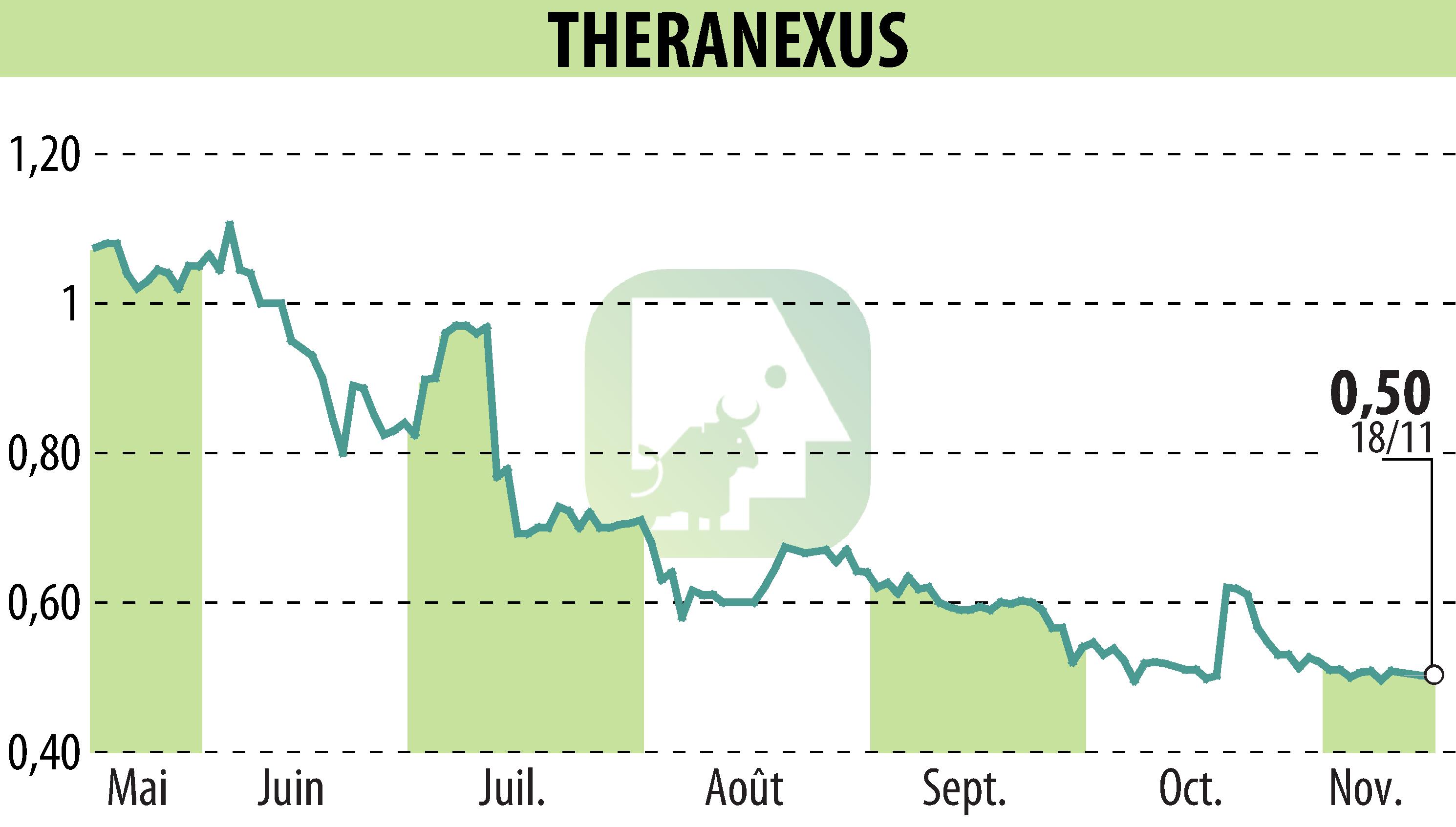
Theranexus and the Beyond Batten Disease Foundation (BBDF) revealed encouraging results from the Phase I/II trial of Batten-1 treatment at the Pediatric Neurology Society Annual Meeting in San Diego. After 18 months of treatment in six young adults with CLN3 Batten disease, results show robust safety and promising efficacy of miglustat, suggesting disease stabilization.
Additional data from compassionate use indicate a potential stabilization of visual acuity in patients, a symptom severely affected in this disease. These results strengthen discussions to integrate visual acuity as a primary endpoint in the next phase III, necessary for the future registration of the drug.
This program generates enthusiasm among clinicians and supports the continued development of Theranexus, continuing its efforts to obtain the resources necessary for this trial.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Theranexus
