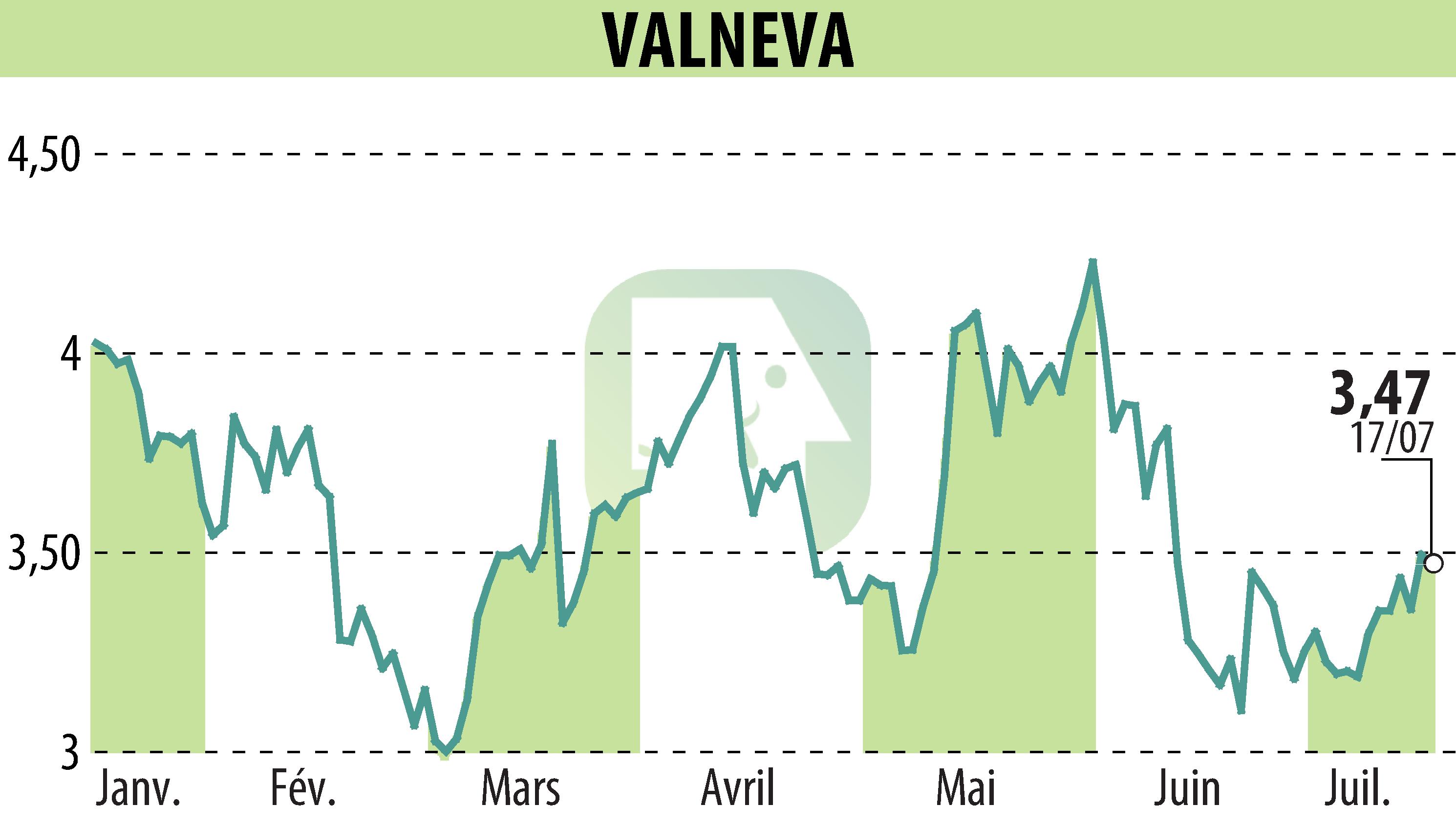
sur VALNEVA (EPA:VLA)
VALOR Phase 3 Trial: Valneva and Pfizer Finalize Primary Lyme Disease Vaccination
Pfizer Inc. and Valneva SE announced the end of the primary vaccination series with the Lyme disease vaccine candidate VLA15 in the Phase 3 VALOR trial. All participants received three doses and will be followed until the end of the 2025 Lyme season.
This milestone is seen as crucial by both companies. Pfizer's Annaliesa Anderson says it's the most advanced program against Lyme disease. Juan Carlos Jaramillo of Valneva highlights the urgency of new approaches to prevent this disease, which is very widespread in the United States and Europe.
VALOR is a randomized, double-blind trial proving the efficacy and safety of VLA15. The results will determine possible authorization by the FDA and EMA, planned for 2026.
VLA15 demonstrated good safety in all age groups tested and no major safety issues have been observed to date. A second ongoing trial aims to establish safety in 5-17 year olds.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
