par MedMira, Inc. (isin : CA58501R1029)
MedMira Receives Canadian Patent for its Unique Quantitative Diagnostic System
HALIFAX, NS / ACCESSWIRE / March 13, 2024 / Today, MedMira Inc. (MedMira) (TSXV:MIR) announces the receipt of a Canadian patent patent (number 2,949,634) for its new innovative and quantitative test system. This is in addition to the U.S. patent (number 11,353,450) received in 2022. Through this new patent, MedMira is to further diversify its patent portfolio and expand on its Rapid Vertical Flow Technology® (RVF) based diagnostic tests. This is a step forward in empowering the Company's strategic vision by offering a rapid multiplexed quantitative diagnostic system from screening to confirmation to monitoring disease progression. The synergies between both patented technologies allow MedMira to continue its corporate aim to provide the market with a highly effective and affordable alternative to the current costly and time-consuming screening and monitoring systems.
"All our rapid tests are based on MedMira's patented RVF Technology® and provide an immediate quality Yes or No answer(s). Our speed in addition to our unique control line, allow for the fastest and high quality answer(s) at a real Point-of-Care setting. While the use of rapid tests have significantly increased over the last years, rapid tests are the first step by providing qualitative result(s). MedMira's vision is to expand this further by adding a unique value with its MIROQ™ system. The Company's RVF Technology® together with the MIROQ™ technology allows health care providers to receive an immediate diagnosis with both qualitative AND quantitative results." said Hermes Chan, CEO of MedMira Inc. "The synergies of both systems provide the highest quality answers within in minutes and at the most economical costs for health care providers. This combination allows for a truly Point-of-Care situation and enables for immediate treatment of patients. Our future vision has already become reality with MIROQ™.
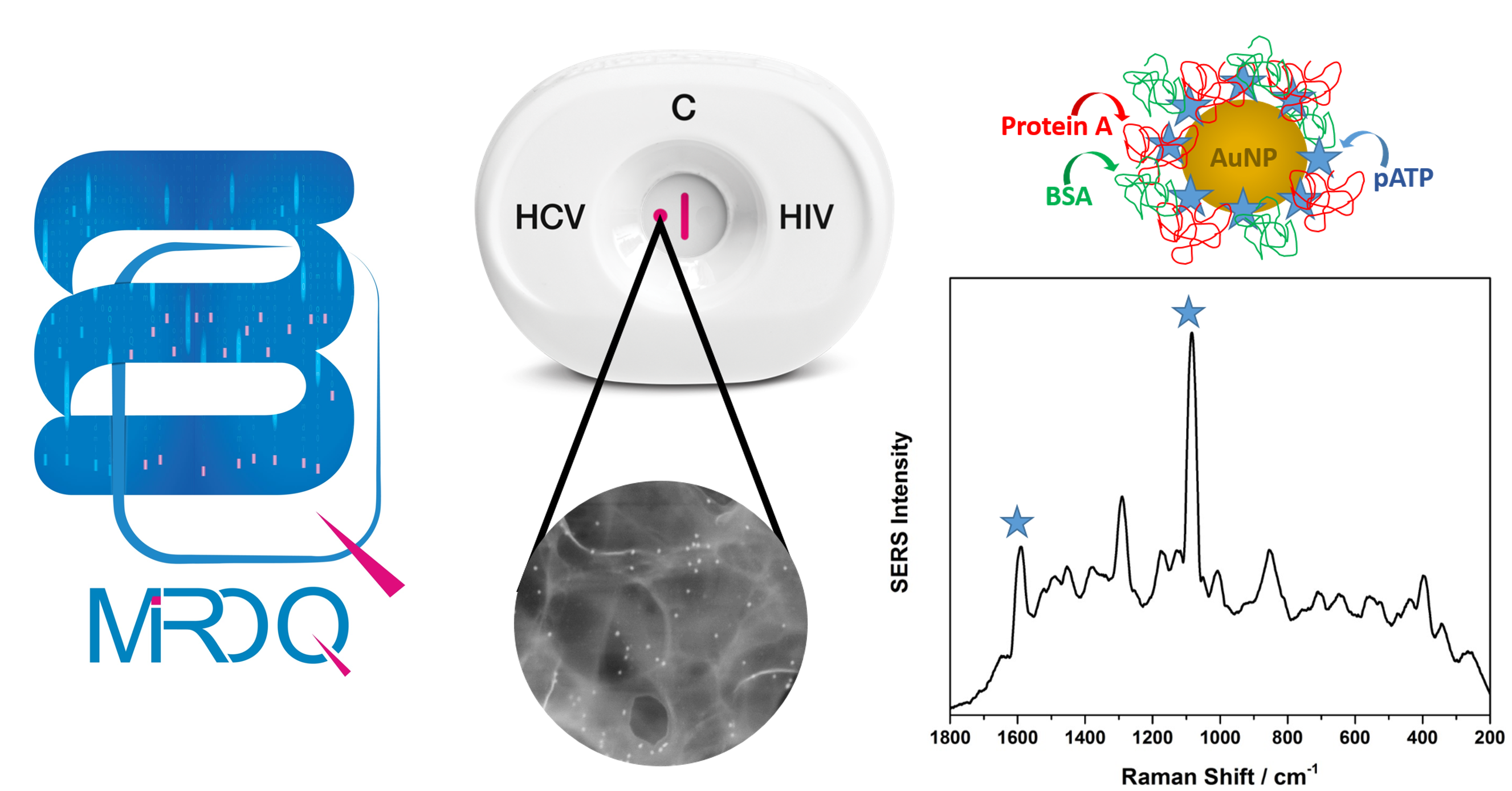
MedMira's latest novel diagnostic system allows for accessible and efficient diagnostic tools for quantitative results in minutes. The user-friendly interface combined with automated interpretation allows for an expansion of MedMira's current RVF-based tests and can provide a pathway to significantly increase the technology's multiplexing abilities. The combination of the RVF and Surface-Enhanced Raman Spectroscopy* (SERS) technology, creates MedMira's patented novel high quality and cost-effective tool for the next generation - MIROQ™. This enables the amplification of the results produced by MedMira's RVF-based rapid tests by creating a unique 3D structure with remarkable reproducibility that is yielded in a linear plot (R2 = 0.98). The new addition to MedMira's patent family creates perfect collaboration to expand its access in both the clinical immunoassay and the Point-of-Care markets, opening new doors into the evolving diagnostic landscape by providing both qualitative and quantitative test results in minutes.
The company developed the first prototype system in 2014 and went through extensive verification and validation performed by our academic partners here in NS, Canada. These findings were published in the Journal of Analytical Chemistry in November 2016 and describe the performance and efficiency of this technology to be on par with traditional expensive laboratory testing solutions which are generally limited to high complexity labs. This patented system with the proprietary build-in data capture and analysis software allows for immediate analysis of any positive (reactive) results within 1 min. This is in contrast to the current laboratory systems that may take from a couple of hours and up to a week to process samples.
*Surface-enhanced Raman Spectroscopy (SERS) is a technique that enhances Raman scattering of molecules embedded on a given surface by several orders of magnitude through the amplification of the electron cloud density around these molecules. Typical SERS signal enhancement factors (EF) are observed between 106 and 1010 times, thus enabling a lower limit of detection and making the tests more sensitive.
About MedMira
MedMira is a leading developer and manufacturer of Rapid Vertical Flow® diagnostics. The Company's tests provide hospitals, labs, clinics and individuals with instant disease diagnosis, such as HIV, Syphilis, Hepatitis, and SARS-CoV-2, in just three easy steps. The Company's tests are sold globally under the REVEAL®, REVEALCOVID-19® , Multiplo® and Miriad® brands. Based on its patented Rapid Vertical Flow® Technology, MedMira's rapid HIV test is the only one in the world to achieve regulatory approvals in Canada, the United States, China and the European Union. MedMira's corporate offices and manufacturing facilities are located in Halifax, Nova Scotia, Canada. For more information visit medmira.com. Follow us on Twitter and LinkedIn.
This news release contains forwardâlooking statements, which involve risk and uncertainties and reflect the Company's current expectation regarding future events, including statements regarding possible regulatory approval, product launch, future growth, and new business opportunities. Actual events could materially differ from those projected herein and depend on a number of factors including, but not limited to, changing market conditions, successful and timely completion of clinical studies, uncertainties related to the regulatory approval process, establishment of corporate alliances and other risks detailed from time to time in the company quarterly filings.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
MedMira Contact
Markus Meile
Chief Financial Officer
MedMira Inc.
ir@medmira.com
SOURCE: MedMira, Inc.
View the original press release on accesswire.com

