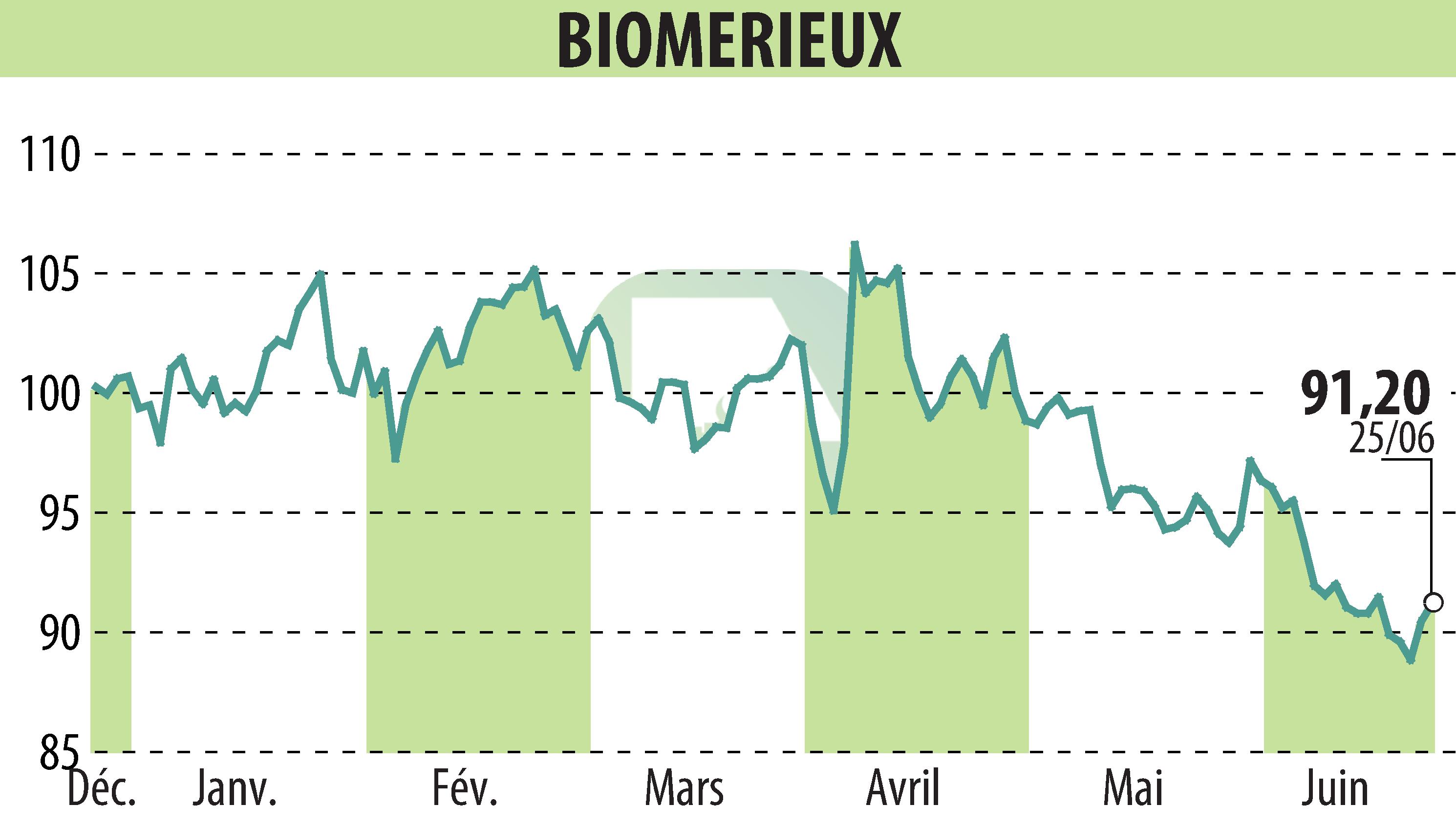
sur BIOMERIEUX (EPA:BIM)
BioMérieux Receives FDA Clearance and CLIA-Waiver for BIOFIRE® SPOTFIRE® R/ST Panel Mini
Marcy-l’Étoile, France — June 26, 2024 — bioMérieux, a leader in in vitro diagnostics, has announced that its BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel Mini has been granted U.S. FDA Special 510(k) clearance and a CLIA-waiver. The COVID-19 pandemic underscored the need for rapid, point-of-care diagnostic tests. The BIOFIRE® SPOTFIRE® system meets this demand.
The BIOFIRE® SPOTFIRE® R/ST Panel Mini is a multiplex PCR test designed for use on the BIOFIRE® SPOTFIRE® system. It identifies five common viral and bacterial pathogens causing respiratory or sore throat infections within 15 minutes. Samples can be collected via nasopharyngeal or throat swabs, depending on the suspected infection.
This system offers flexibility, allowing clinicians to choose between a large panel testing for up to 15 pathogens or a smaller one for five. This approach enhances diagnostic yield and reduces the need for cultures. The R/ST Panel Mini is the fourth panel in its range to receive these regulatory clearances.
Jennifer Zinn, Executive VP of Clinical Operations, highlighted the system's ability to test for common respiratory viruses and Streptococcus A from a single swab. This innovation aims to meet the growing needs of decentralized point-of-care settings, allowing clinicians to make quick, informed decisions about patient treatment. The panel will be available in the U.S. from the third quarter of 2024.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de BIOMERIEUX
