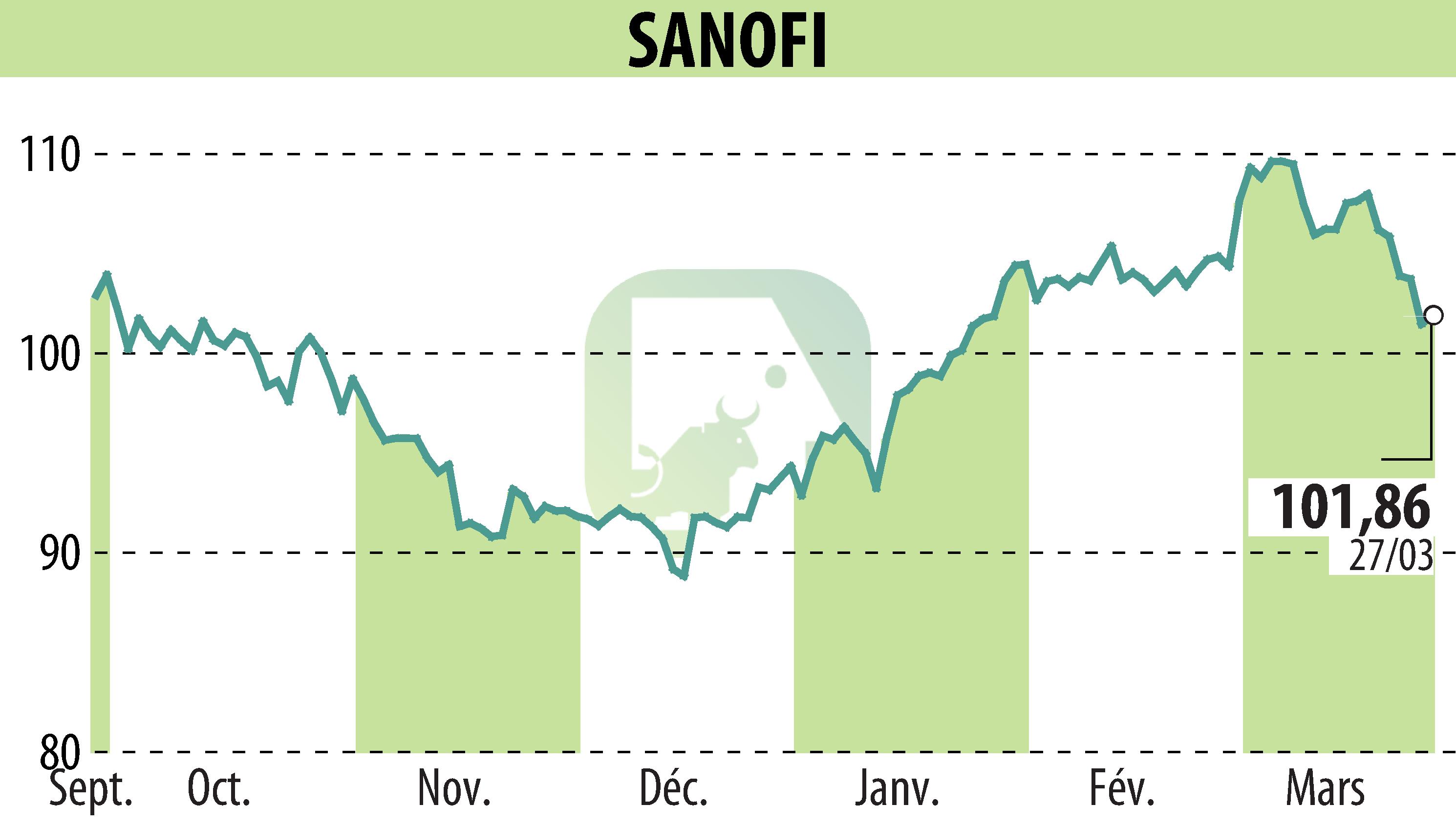
sur SANOFI-AVENTIS (EPA:SAN)
Dupixent Approved in Japan as First Biologic for COPD
Sanofi-Aventis announced that Japan's Ministry of Health, Labour and Welfare has approved Dupixent (dupilumab) as the first biological treatment for chronic obstructive pulmonary disease (COPD) in adults inadequately controlled by existing therapies. This decision follows approvals in the EU, China, and the US.
The approval is based on phase 3 trials, which demonstrated Dupixent's efficacy in reducing exacerbations and improving lung function in COPD patients with elevated eosinophils. The safety profile was consistent with previous findings, with injection site reactions being the most common adverse event.
In addition to COPD, Dupixent is approved in Japan for conditions like atopic dermatitis and asthma. Globally, Dupixent is approved in over 45 countries for COPD, marking a significant step in the treatment of chronic diseases with underlying type 2 inflammation.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
