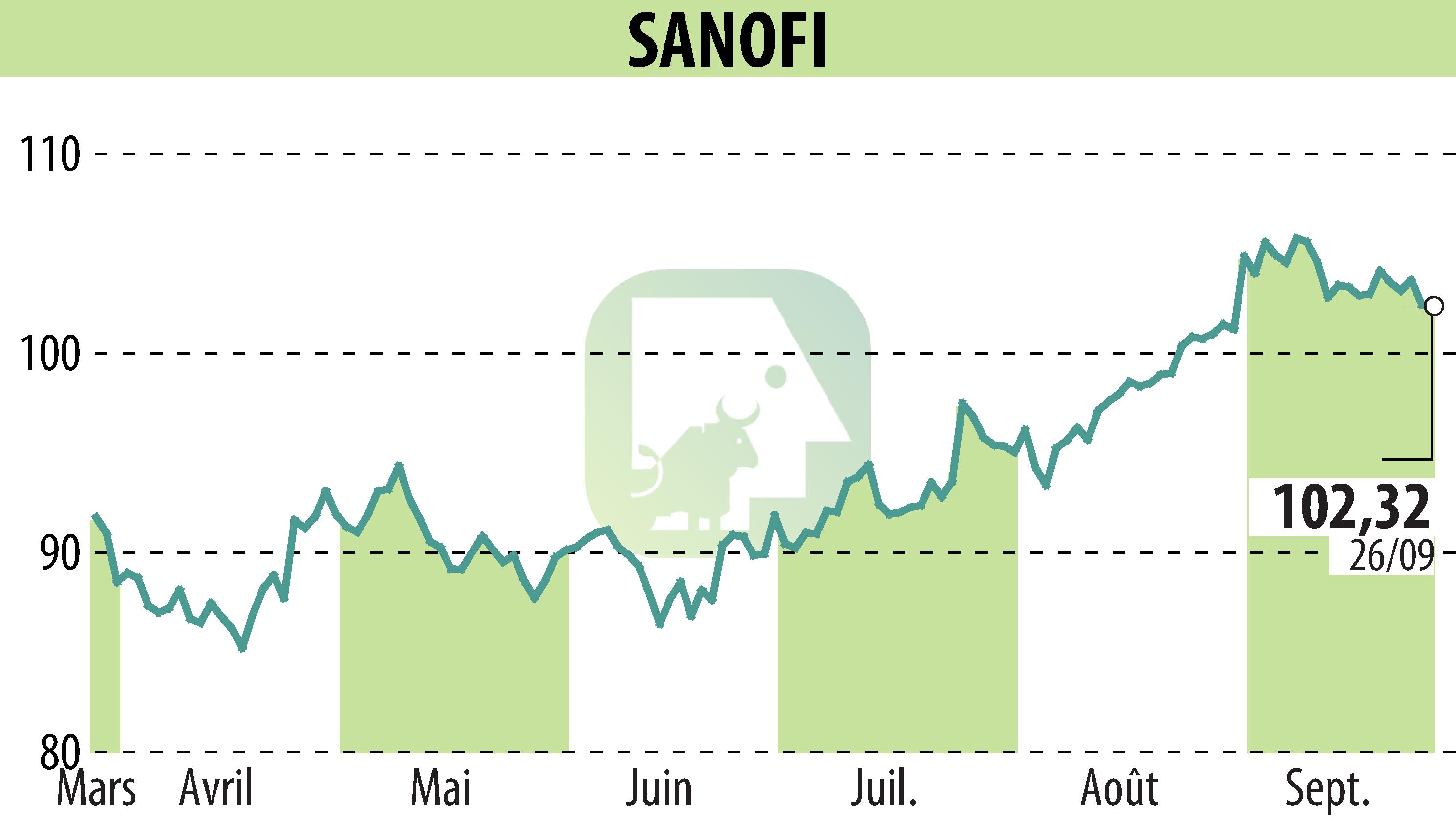
sur SANOFI-AVENTIS (EPA:SAN)
Dupixent Authorized in China as First Biologic Medicine for COPD Patients
The National Medical Products Administration (NMPA) in China has approved Dupixent (dupilumab) as an add-on treatment for adults with uncontrolled chronic obstructive pulmonary disease (COPD) characterized by raised blood eosinophils. This approval marks the first instance of a biologic medicine being available for COPD patients in China, following EU approval earlier this year.
Based on two pivotal phase 3 studies, Dupixent significantly reduced COPD exacerbations and improved lung function and quality of life. COPD, the most common chronic respiratory disease in China, is a key focus in the government’s Healthy China 2030 public health plan.
With this, Dupixent is now approved for four indications across respiratory and dermatological diseases in China. The approval brings a novel treatment option to millions of COPD patients who remain inadequately controlled even after maximum inhaled therapy.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
