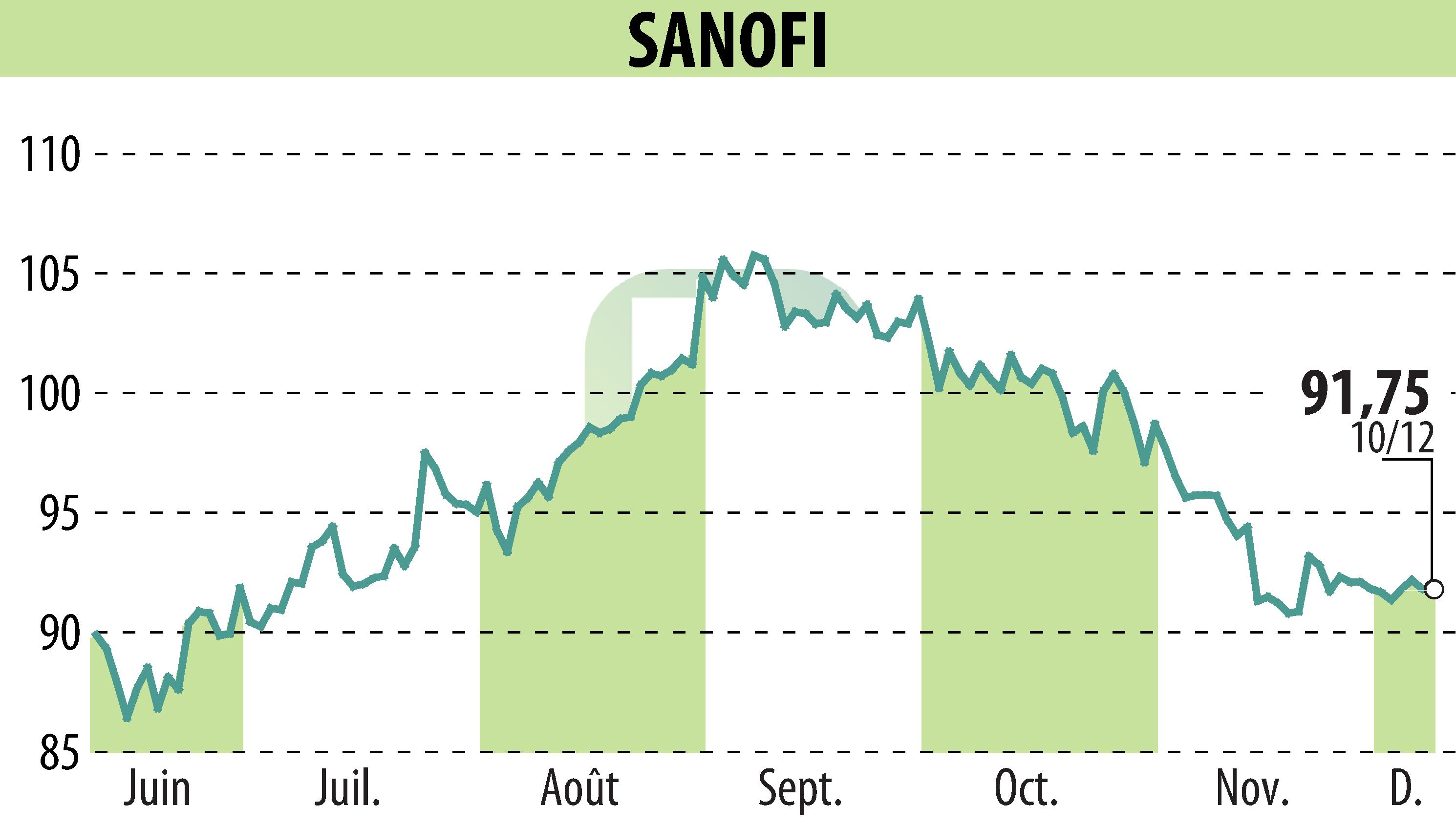
sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's two combination vaccines against influenza and COVID-19 receive expedited review
Sanofi has received accelerated review from the FDA for two combination vaccine candidates to prevent influenza and COVID-19 infections. Intended for adults 50 years of age and older, the vaccines combine formulations that are already approved and have proven efficacy. The first vaccine combines Fluzone High-Dose with the Novavax COVID-19 vaccine, while the second combines Flublok with the same vaccine. Phase I/II clinical studies are underway to evaluate their safety and immune response.
Combination vaccines aim to simplify vaccination by reducing the number of injections needed and decreasing the risk of hospitalization. A meta-analysis suggests that these vaccines would increase adult acceptance of boosters.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
